Single-Molecule Tethered Particle Motion: Stepwise Analyses of Site-Specific DNA Recombination
- PMID: 30424148
- PMCID: PMC6187709
- DOI: 10.3390/mi9050216
Single-Molecule Tethered Particle Motion: Stepwise Analyses of Site-Specific DNA Recombination
Abstract
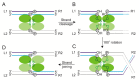
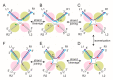
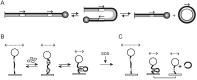
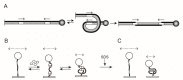
Tethered particle motion/microscopy (TPM) is a biophysical tool used to analyze changes in the effective length of a polymer, tethered at one end, under changing conditions. The tether length is measured indirectly by recording the Brownian motion amplitude of a bead attached to the other end. In the biological realm, DNA, whose interactions with proteins are often accompanied by apparent or real changes in length, has almost exclusively been the subject of TPM studies. TPM has been employed to study DNA bending, looping and wrapping, DNA compaction, high-order DNA⁻protein assembly, and protein translocation along DNA. Our TPM analyses have focused on tyrosine and serine site-specific recombinases. Their pre-chemical interactions with DNA cause reversible changes in DNA length, detectable by TPM. The chemical steps of recombination, depending on the substrate and the type of recombinase, may result in a permanent length change. Single molecule TPM time traces provide thermodynamic and kinetic information on each step of the recombination pathway. They reveal how mechanistically related recombinases may differ in their early commitment to recombination, reversibility of individual steps, and in the rate-limiting step of the reaction. They shed light on the pre-chemical roles of catalytic residues, and on the mechanisms by which accessory proteins regulate recombination directionality.
Keywords: Cre; Flp; serine recombinases; single molecule analysis; site-specific recombination; tethered particle motion; tyrosine recombinases; ϕC31integrase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kovari D.T., Yan Y., Finzi L., Dunlap D. Tethered Particle Motion: An easy technique for probing DNA topology and interactions with transcription factors. In: Peterman E.J.G., editor. Single Molecule Analysis: Methods and Protocols, Methos in Molecular Biology. Volume 1665. Springer + Business Media LLC; New York, NY, USA: 2018. pp. 5–17. - PMC - PubMed
-
- Han L., Lui B.H., Blumberg S., Beausang J.F., Nelson P.C., Phillips R. Mathematics of DNA Structure, Function and Interactions (Dedicated to Nick Cozzarelli) Springer; New York, NY, USA: 2007. Calibration of tethered particle motion experiments.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

