AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling
- PMID: 30424476
- PMCID: PMC6274864
- DOI: 10.3390/ijms19113558
AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling
Abstract
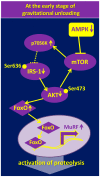
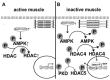
Molecular mechanisms that trigger disuse-induced postural muscle atrophy as well as myosin phenotype transformations are poorly studied. This review will summarize the impact of 5' adenosine monophosphate -activated protein kinase (AMPK) activity on mammalian target of rapamycin complex 1 (mTORC1)-signaling, nuclear-cytoplasmic traffic of class IIa histone deacetylases (HDAC), and myosin heavy chain gene expression in mammalian postural muscles (mainly, soleus muscle) under disuse conditions, i.e., withdrawal of weight-bearing from ankle extensors. Based on the current literature and the authors' own experimental data, the present review points out that AMPK plays a key role in the regulation of signaling pathways that determine metabolic, structural, and functional alternations in skeletal muscle fibers under disuse.
Keywords: AMPK; HDAC4/5; MyHC I(β), motor endplate remodeling; hindlimb suspension; mechanical unloading; p70S6K; soleus muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Grigor’ev A.I., Kozlovskaia I.B., Shenkman B.S. The role of support afferents in organisation of the tonic muscle system. Rossiiskii fiziologicheskii zhurnal imeni IM Sechenova. 2004;90:508–521. - PubMed
-
- Kozlovskaia I.B., Grigor’eva L.S., Gevlich G.I. Comparative analysis of the effect of weightlessness and its model on the velocity-strength properties and tonus of human skeletal muscles. Kosm. Biol. Aviakosm. Med. 1984;18:22–26. - PubMed
-
- Oganov V.S., Skuratova S.A., Murashko L.M., Guba F., Takach O. Effect of short-term space flights on physiological properties and composition of myofibrillar proteins of the skeletal muscles of rats. Kosm. Biol. Aviakosm. Med. 1988;22:50–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

