Characterizing Longitudinal Changes in the Impedance Spectra of In-Vivo Peripheral Nerve Electrodes
- PMID: 30424513
- PMCID: PMC6266965
- DOI: 10.3390/mi9110587
Characterizing Longitudinal Changes in the Impedance Spectra of In-Vivo Peripheral Nerve Electrodes
Abstract
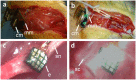
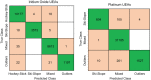
Characterizing the aging processes of electrodes in vivo is essential in order to elucidate the changes of the electrode⁻tissue interface and the device. However, commonly used impedance measurements at 1 kHz are insufficient for determining electrode viability, with measurements being prone to false positives. We implanted cohorts of five iridium oxide (IrOx) and six platinum (Pt) Utah arrays into the sciatic nerve of rats, and collected the electrochemical impedance spectroscopy (EIS) up to 12 weeks or until array failure. We developed a method to classify the shapes of the magnitude and phase spectra, and correlated the classifications to circuit models and electrochemical processes at the interface likely responsible. We found categories of EIS characteristic of iridium oxide tip metallization, platinum tip metallization, tip metal degradation, encapsulation degradation, and wire breakage in the lead. We also fitted the impedance spectra as features to a fine-Gaussian support vector machine (SVM) algorithm for both IrOx and Pt tipped arrays, with a prediction accuracy for categories of 95% and 99%, respectively. Together, this suggests that these simple and computationally efficient algorithms are sufficient to explain the majority of variance across a wide range of EIS data describing Utah arrays. These categories were assessed over time, providing insights into the degradation and failure mechanisms for both the electrode⁻tissue interface and wire bundle. Methods developed in this study will allow for a better understanding of how EIS can characterize the physical changes to electrodes in vivo.
Keywords: Utah electrode arrays; electrode–tissue interface; impedance; peripheral nerves.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Caldwell R., Sharma R., Takmakov P., Street M.G., Solzbacher F., Tathireddy P., Rieth L. Neural Electrode Resilience against Dielectric Damage May Be Improved by Use of Highly Doped Silicon as a Conductive Material. J. Neurosci. Methods. 2018;293:210–225. doi: 10.1016/j.jneumeth.2017.10.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

