Anticoagulant Properties of a Green Algal Rhamnan-type Sulfated Polysaccharide and Its Low-molecular-weight Fragments Prepared by Mild Acid Degradation
- PMID: 30424528
- PMCID: PMC6266706
- DOI: 10.3390/md16110445
Anticoagulant Properties of a Green Algal Rhamnan-type Sulfated Polysaccharide and Its Low-molecular-weight Fragments Prepared by Mild Acid Degradation
Abstract
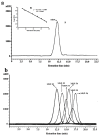
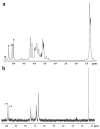
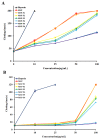
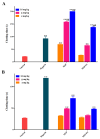
The active sulfated polysaccharide from seaweed possesses important pharmaceutical and biomedical potential. In the study, Monostroma sulfated polysaccharide (MSP) was obtained from Monostroma angicava, and the low-molecular-weight fragments of MSP (MSP-Fs: MSP-F1⁻MSP-F6) were prepared by controlled acid degradation. The molecular weights of MSP and MSP-F1⁻MSP-F6 were 335 kDa, 240 kDa, 90 kDa, 40 kDa, 24 kDa, 12 kDa, and 6.8 kDa, respectively. The polysaccharides were sulfated rhamnans that consisted of →3)-α-l-Rhap-(1→ and →2)-α-l-Rhap-(1→ units with partial sulfation at C-2 of →3)-α-l-Rhap-(1→ and C-3 of →2)-α-l-Rhap-(1→. Anticoagulant properties in vitro of MSP and MSP-F1⁻MSP-F6 were evaluated by studying the activated partial thromboplastin time, thrombin time, and prothrombin time. Anticoagulant activities in vivo of MSP and MSP-F4 were further evaluated; their fibrin(ogen)olytic activities in vivo and thrombolytic properties in vitro were also assessed by D-dimer, fibrin degradation products, plasminogen activator inhibitior-1, and clot lytic rate assays. The results showed that MSP and MSP-F1⁻MSP-F4 with molecular weights of 24⁻240 kDa had strong anticoagulant activities. A decrease in the molecular weight of MSP-Fs was accompanied by a decrease in the anticoagulant activity, and higher anticoagulant activity requires a molecular weight of over 12 kDa. MSP and MSP-F4 possessed strong anticoagulant activities in vivo, as well as high fibrin(ogen)olytic and thrombolytic activities. MSP and MSP-F4 have potential as drug or helpful food supplements for human health.
Keywords: anticoagulant activity; depolymerization; fibrin(ogen)olytic activity; low-molecular-weight fractions; sulfated polysaccharide; thrombolytic activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Gupta S., Abughannam N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food. Sci. Technol. 2011;22:315–326. doi: 10.1016/j.tifs.2011.03.011. - DOI
-
- Wijesekara I., Pangestuti R., Kim S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011;84:14–21. doi: 10.1016/j.carbpol.2010.10.062. - DOI
-
- Lee J.B., Yamagaki T., Maeda M., Nakanishi H. Rhamnan sulfate from cell walls of Monostroma latissimum. Phytochemistry. 1998;48:921–925. doi: 10.1016/S0031-9422(97)00927-8. - DOI
-
- Lee J.B., Koizumi S., Hayashi K., Hayashi T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr. Polym. 2010;81:572–577. doi: 10.1016/j.carbpol.2010.03.014. - DOI
MeSH terms
Substances
Grants and funding
- 41476108/National Natural Science Foundation of China
- 2018YFC0310900/National Key Research and Development Program of China
- U1606403/NSFC-Shandong Joint Fund for Marine Science Research Centers
- 2016ASKJ08 and 2015ASKJ02/Scientific and Technological Innovation Project of Qingdao National Laboratory for Marine Science and Technology
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

