Improving care at scale: process evaluation of a multi-component quality improvement intervention to reduce mortality after emergency abdominal surgery (EPOCH trial)
- PMID: 30424818
- PMCID: PMC6233578
- DOI: 10.1186/s13012-018-0823-9
Improving care at scale: process evaluation of a multi-component quality improvement intervention to reduce mortality after emergency abdominal surgery (EPOCH trial)
Erratum in
-
Correction to: Improving care at scale: process evaluation of a multi-component quality improvement intervention to reduce mortality after emergency abdominal surgery (EPOCH trial).Implement Sci. 2018 Dec 10;13(1):148. doi: 10.1186/s13012-018-0840-8. Implement Sci. 2018. PMID: 30526645 Free PMC article.
Abstract
Background: Improving the quality and safety of perioperative care is a global priority. The Enhanced Peri-Operative Care for High-risk patients (EPOCH) trial was a stepped-wedge cluster randomised trial of a quality improvement (QI) programme to improve 90-day survival for patients undergoing emergency abdominal surgery in 93 hospitals in the UK National Health Service.
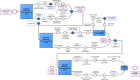
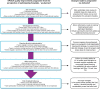
Methods: The aim of this process evaluation is to describe how the EPOCH intervention was planned, delivered and received, at both cluster and local hospital levels. The QI programme comprised of two interventions: a care pathway and a QI intervention to aid pathway implementation, focussed on stakeholder engagement, QI teamwork, data analysis and feedback and applying the model for improvement. Face-to-face training and online resources were provided to support senior clinicians in each hospital (QI leads) to lead improvement. For this evaluation, we collated programme activity data, administered an exit questionnaire to QI leads and collected ethnographic data in six hospitals. Qualitative data were analysed with thematic or comparative analysis; quantitative data were analysed using descriptive statistics.
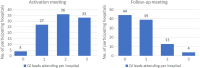
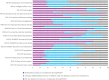
Results: The EPOCH trial did not demonstrate any improvement in survival or length of hospital stay. Whilst the QI programme was delivered as planned at the cluster level, self-assessed intervention fidelity at the hospital level was variable. Seventy-seven of 93 hospitals responded to the exit questionnaire (60 from a single QI lead response on behalf of the team); 33 respondents described following the QI intervention closely (35%) and there were only 11 of 37 care pathway processes that > 50% of respondents reported attempting to improve. Analysis of qualitative data suggests QI leads were often attempting to deliver the intervention in challenging contexts: the social aspects of change such as engaging colleagues were identified as important but often difficult and clinicians frequently attempted to lead change with limited time or organisational resources.
Conclusions: Significant organisational challenges faced by QI leads shaped their choice of pathway components to focus on and implementation approaches taken. Adaptation causing loss of intervention fidelity was therefore due to rational choices made by those implementing change within constrained contexts. Future large-scale QI programmes will need to focus on dedicating local time and resources to improvement as well as on training to develop QI capabilities.
Epoch trial registration: ISRCTN80682973 https://doi.org/10.1186/ISRCTN80682973 Registered 27 February 2014 and Lancet protocol 13PRT/7655.
Keywords: Complex interventions; Emergency surgery; Evaluation; Quality improvement.
Conflict of interest statement
Ethics approval and consent to participate
The EPOCH trial was approved by the Research Ethics Committee of the National Health Service (REC reference 13/EM/0415). Completion of the EPOCH trial exit questionnaire implied consent to participate; written information was provided at the start of the online questionnaire about the purpose of the questionnaire, the voluntary nature of participation and assurances that no individual or hospital level data would be disclosed.
Consent for publication
Not applicable.
Competing interests
RP holds research grants and has given lectures and/or performed consultancy work for Nestle Health Sciences, BBraun, Medtronic and Edwards Lifesciences, and is a member of the Associate editorial board of the British Journal of Anaesthesia. All other authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

