MTORC1/2 Inhibition as a Therapeutic Strategy for PIK3CA Mutant Cancers
- PMID: 30425131
- PMCID: PMC6363831
- DOI: 10.1158/1535-7163.MCT-18-0510
MTORC1/2 Inhibition as a Therapeutic Strategy for PIK3CA Mutant Cancers
Abstract
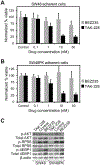
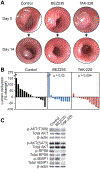
PIK3CA mutations are common in clinical molecular profiling, yet an effective means to target these cancers has yet to be developed. MTORC1 inhibitors are often used off-label for patients with PIK3CA mutant cancers with only limited data to support this approach. Here we describe a cohort of patients treated with cancers possessing mutations activating the PI3K signaling cascade with minimal benefit to treatment with the MTORC1 inhibitor everolimus. Previously, we demonstrated that dual PI3K/mTOR inhibition could decrease proliferation, induce differentiation, and result in a treatment response in APC and PIK3CA mutant colorectal cancer. However, reactivation of AKT was identified, indicating that the majority of the benefit may be secondary to MTORC1/2 inhibition. TAK-228, an MTORC1/2 inhibitor, was compared with dual PI3K/mTOR inhibition using BEZ235 in murine colorectal cancer spheroids. A reduction in spheroid size was observed with TAK-228 and BEZ235 (-13% and -14%, respectively) compared with an increase of >200% in control (P < 0.001). These spheroids were resistant to MTORC1 inhibition. In transgenic mice possessing Pik3ca and Apc mutations, BEZ235 and TAK-228 resulted in a median reduction in colon tumor size of 19% and 20%, respectively, with control tumors having a median increase of 18% (P = 0.02 and 0.004, respectively). This response correlated with a decrease in the phosphorylation of 4EBP1 and RPS6. MTORC1/2 inhibition is sufficient to overcome resistance to everolimus and induce a treatment response in PIK3CA mutant colorectal cancers and deserves investigation in clinical trials and in future combination regimens.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
References
-
- Cancer Facts & Figures 2017. American Cancer Society: Atlanta, 2017.
-
- Deming D, Holen K. KRAS Mutation Analysis Prior to EGFR-Directed Therapy for Metastatic Colorectal Cancer: A Review and Cost Analysis. Current Cancer Therapy Reviews 2010;6:256–261.
-
- Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091–2096. - PubMed
-
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–1034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

