An ultra-compact integrated system for brain activity recording and stimulation validated over cortical slow oscillations in vivo and in vitro
- PMID: 30425252
- PMCID: PMC6233193
- DOI: 10.1038/s41598-018-34560-y
An ultra-compact integrated system for brain activity recording and stimulation validated over cortical slow oscillations in vivo and in vitro
Abstract
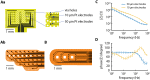
The understanding of brain processing requires monitoring and exogenous modulation of neuronal ensembles. To this end, it is critical to implement equipment that ideally provides highly accurate, low latency recording and stimulation capabilities, that is functional for different experimental preparations and that is highly compact and mobile. To address these requirements, we designed a small ultra-flexible multielectrode array and combined it with an ultra-compact electronic system. The device consists of a polyimide microelectrode array (8 µm thick and with electrodes measuring as low as 10 µm in diameter) connected to a miniaturized electronic board capable of amplifying, filtering and digitalizing neural signals and, in addition, of stimulating brain tissue. To evaluate the system, we recorded slow oscillations generated in the cerebral cortex network both from in vitro slices and from in vivo anesthetized animals, and we modulated the oscillatory pattern by means of electrical and visual stimulation. Finally, we established a preliminary closed-loop algorithm in vitro that exploits the low latency of the electronics (<0.5 ms), thus allowing monitoring and modulating emergent cortical activity in real time to a desired target oscillatory frequency.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Caton R. Electrical Currents of the Brain. J. Nerv. Ment. Dis. 1875;2:610.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

