Structure and oligomerization of the periplasmic domain of GspL from the type II secretion system of Pseudomonas aeruginosa
- PMID: 30425318
- PMCID: PMC6233222
- DOI: 10.1038/s41598-018-34956-w
Structure and oligomerization of the periplasmic domain of GspL from the type II secretion system of Pseudomonas aeruginosa
Abstract
The ability of bacteria to infect a host relies in part on the secretion of molecular virulence factors across the cell envelope. Pseudomonas aeruginosa, a ubiquitous environmental bacterium causing opportunistic infections in humans, employs the type II secretion system (T2SS) to transport effector proteins across its cellular envelope as part of a diverse array of virulence strategies. General secretory pathway protein L (GspL) is an essential inner-membrane component of the T2SS apparatus, and is thought to facilitate transduction of the energy from ATP hydrolysis in the cytoplasm to the periplasmic components of the system. However, our incomplete understanding of the assembly principles of the T2SS machinery prevents the mechanistic deconvolution of T2SS-mediated protein secretion. Here we show via two crystal structures that the periplasmic ferredoxin-like domain of GspL (GspLfld) is a dimer stabilized by hydrophobic interactions, and that this interface may allow significant interdomain plasticity. The general dimerization mode of GspLfld is shared with GspL from Vibrio parahaemolyticus suggesting a conserved oligomerization mode across the GspL family. Furthermore, we identified a tetrameric form of the complete periplasmic segment of GspL (GspLperi) which indicates that GspL may be able to adopt multiple oligomeric states as part of its dynamic role in the T2SS apparatus.
Conflict of interest statement
The authors declare no competing interests.
Figures

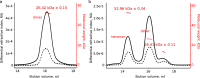



Similar articles
-
Architecture, Function, and Substrates of the Type II Secretion System.EcoSal Plus. 2019 Feb;8(2):10.1128/ecosalplus.ESP-0034-2018. doi: 10.1128/ecosalplus.ESP-0034-2018. EcoSal Plus. 2019. PMID: 30767847 Free PMC article. Review.
-
Direct interactions between the secreted effector and the T2SS components GspL and GspM reveal a new effector-sensing step during type 2 secretion.J Biol Chem. 2018 Dec 14;293(50):19441-19450. doi: 10.1074/jbc.RA117.001127. Epub 2018 Oct 18. J Biol Chem. 2018. PMID: 30337370 Free PMC article.
-
Dynamic interplay between the periplasmic and transmembrane domains of GspL and GspM in the type II secretion system.PLoS One. 2013 Nov 1;8(11):e79562. doi: 10.1371/journal.pone.0079562. eCollection 2013. PLoS One. 2013. PMID: 24223969 Free PMC article.
-
Crystal structure of the full-length ATPase GspE from the Vibrio vulnificus type II secretion system in complex with the cytoplasmic domain of GspL.J Struct Biol. 2014 Sep;187(3):223-235. doi: 10.1016/j.jsb.2014.07.006. Epub 2014 Aug 1. J Struct Biol. 2014. PMID: 25092625 Free PMC article.
-
Bacterial Type II Secretion System and Its Mitochondrial Counterpart.mBio. 2023 Apr 25;14(2):e0314522. doi: 10.1128/mbio.03145-22. Epub 2023 Mar 27. mBio. 2023. PMID: 36971557 Free PMC article. Review.
Cited by
-
Architecture, Function, and Substrates of the Type II Secretion System.EcoSal Plus. 2019 Feb;8(2):10.1128/ecosalplus.ESP-0034-2018. doi: 10.1128/ecosalplus.ESP-0034-2018. EcoSal Plus. 2019. PMID: 30767847 Free PMC article. Review.
-
In through the Out Door: A Functional Virulence Factor Secretion System Is Necessary for Phage Infection in Ralstonia solanacearum.mBio. 2022 Dec 20;13(6):e0147522. doi: 10.1128/mbio.01475-22. Epub 2022 Oct 31. mBio. 2022. PMID: 36314808 Free PMC article.
-
Impact of AbaI mutation on virulence, biofilm development, and antibiotic susceptibility in Acinetobacter baumannii.Sci Rep. 2024 Sep 14;14(1):21521. doi: 10.1038/s41598-024-72740-1. Sci Rep. 2024. PMID: 39277662 Free PMC article.
-
Bacterial secretins: Mechanisms of assembly and membrane targeting.Protein Sci. 2020 Apr;29(4):893-904. doi: 10.1002/pro.3835. Epub 2020 Feb 19. Protein Sci. 2020. PMID: 32020694 Free PMC article. Review.
-
Protein Export via the Type III Secretion System of the Bacterial Flagellum.Biomolecules. 2021 Jan 29;11(2):186. doi: 10.3390/biom11020186. Biomolecules. 2021. PMID: 33572887 Free PMC article. Review.
References
-
- Thomassin J, Moreno JS, Guilvout I, Nhieu GT. Van. The trans-envelope architecture and function of the type 2 secretion system: new insights raising new questions. Mol. Microbiol. 2017;105:211–226. - PubMed
-
- WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET... (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

