The efficacy and safety of immune checkpoint inhibitor combination therapy in lung cancer: a systematic review and meta-analysis
- PMID: 30425525
- PMCID: PMC6204847
- DOI: 10.2147/OTT.S177318
The efficacy and safety of immune checkpoint inhibitor combination therapy in lung cancer: a systematic review and meta-analysis
Abstract
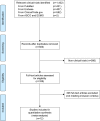
The value of immune checkpoint inhibitor (ICI) combination therapy for patients with lung cancer remains unclear. We conducted a meta-analysis using PubMed, Embase, and ClinicalTrials.gov databases to identify eligible randomized controlled trials (RCTs) that might provide a reference for clinical practice. The selection criteria were defined according to the population, intervention, comparison, outcome and study design (PICOS) framework. In all, 12 RCTs with 5,989 patients were included in this meta-analysis. Our results showed that ICI combination therapy was significantly associated with the improvement of overall response rate (ORR) (RR =1.44 [95% CI 1.19, 1.74], P=0.0002), progression-free survival (PFS) (HR =0.67 [95% CI 0.59, 0.77], P<0.00001), and OS (HR =0.81 [95% CI 0.70, 0.95], P=0.008) in lung cancer. In subgroup analyses, combination ICI therapy significantly prolonged OS in non-small-cell lung cancer (NSCLC) patients (HR =0.80 [95% CI 0.73, 0.88], P<0.00001) but not in SCLC (HR =0.94 [95% CI 0.82, 1.08], P=0.40) patients. Data suggested that PD-1 inhibitors had higher efficacy and safety profiles than PD-L1 and CTLA-4 inhibitors in combination ICI therapy for lung cancer patients. Furthermore, tolerability analysis revealed higher incidences of grade ≥3 AEs, fatigue, and increased transaminases from combination ICI therapy. In conclusion, our meta-analysis indicated that combination ICI therapy should be considered in clinical practice and future study designs for NSCLC patients. However, the current data do not support the large-scale clinical application of combination ICI therapy in SCLC patients.
Keywords: chemoradiotherapy; combination therapy; immune checkpoint inhibitor; lung cancer; meta-analysis.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures








References
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials

