Ketamine Reverses Lateral Habenula Neuronal Dysfunction and Behavioral Immobility in the Forced Swim Test Following Maternal Deprivation in Late Adolescent Rats
- PMID: 30425634
- PMCID: PMC6218426
- DOI: 10.3389/fnsyn.2018.00039
Ketamine Reverses Lateral Habenula Neuronal Dysfunction and Behavioral Immobility in the Forced Swim Test Following Maternal Deprivation in Late Adolescent Rats
Abstract
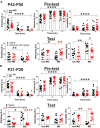
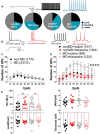
Mounting evidence suggests that the long-term effects of adverse early life stressors on vulnerability to drug addiction and mood disorders are related to dysfunction of brain monoaminergic signaling in reward circuits. Recently, there has been a growing interest in the lateral habenula (LHb) as LHb dysfunction is linked to the development of mental health disorders through monoaminergic dysregulation within brain reward/motivational circuits and may represent a critical target for novel anti-depressants, such as ketamine. Here, we show that maternal deprivation (MD), a severe early life stressor, increases LHb intrinsic excitability and LHb bursting activity, and is associated with the development of increased immobility in the forced swim test (FST) in late-adolescent male rats. A single in vivo injection of ketamine is sufficient to exert prolonged antidepressant effects through reversal of this early life stress-induced LHb neuronal dysfunction and the response in the FST. Our assessment of ketamine's long-lasting beneficial effects on reversal of MD-associated changes in LHb neuronal function and behavior highlights the critical role of the LHb in pathophysiology of depression associated with severe early life stress and in response to novel fast-acting antidepressants.
Keywords: LHb; depression; early life stress; forced swim test; intrinsic excitability; ketamine; lateral habenula; maternal deprivation.
Figures
References
-
- Carlson P. J., Diazgranados N., Nugent A. C., Ibrahim L., Luckenbaugh D. A., Brutsche N., et al. . (2013). Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol. Psychiatry 73, 1213–1221. 10.1016/j.biopsych.2013.02.008 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

