Heterologous Boost Following Mycobacterium bovis BCG Reduces the Late Persistent, Rather Than the Early Stage of Intranasal Tuberculosis Challenge Infection
- PMID: 30425711
- PMCID: PMC6218689
- DOI: 10.3389/fimmu.2018.02439
Heterologous Boost Following Mycobacterium bovis BCG Reduces the Late Persistent, Rather Than the Early Stage of Intranasal Tuberculosis Challenge Infection
Abstract
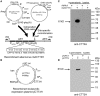
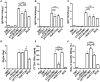
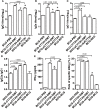
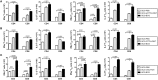
Adults are the leading population affected by tuberculosis (TB) epidemic and death. Developing an effective vaccine against adult TB is urgently needed. Mycobacterium bovis Bacillus Calmette-Guerin (BCG) prime-heterologous boost strategy has been explored extensively to protect adults against primary TB infection, but the majority of experimental regimens have not improved the protection primed by the BCG vaccine. The reason attributed to the failure remains unknown. In this study, CTT3H-based vaccines, namely DMT adjuvanted CTT3H subunit or DNA vaccine (pCTT3H-DMT), and recombinant adenovirus rAdCTT3H were constructed. Protective efficacy and immunogenicity of BCG prime-CTT3H based boosters were compared in C57BL/c mice models of primary or late persistent TB infection. Similar protective efficacy against early intranasal infection was provided by different CTT3H-based vaccines alone in vaccinated mice, and their protection was inferior to that of the BCG vaccine. In addition, CTT3H-based heterologous boosters did not enhance the protection conferred by the BCG vaccine against primary infection. However, all of these three boosters provided stronger protection against late persistent TB infection than BCG alone, regardless of vaccine types. Although BCG prime-boosters elicited Th1-biased responses to the antigen CTT3H, the number of CTT3H-sepcific IFN-γ-expressing TEM (CD62LloCD44hi) and IL-2-expressing TCM (CD62LhiCD44hi) cells in the spleen was not improved before exposure to Mycobacterium tuberculosis infection. In contrast, IFN-γ+ TEM and IL-2+ TCM cells in spleens, especially in lungs were significantly increased in BCG prime-boosters after exposure vaccination. Our results indicate that BCG prime-boost strategy might be a promising measure for the prevention against late persistent TB infection by induction of IFN-γ+ TEM and IL-2+ TCM cells in the lung, which can be used as alternative biomarkers for guiding the clinical practice and future development of TB vaccine for adults.
Keywords: BCG; early stage of tuberculosis; heterologous boost; late persistent tuberculosis; memory T cells.
Figures



Similar articles
-
Prime-boost vaccination strategy with bacillus Calmette-Guérin (BCG) and liposomized alpha-crystalline protein 1 reinvigorates BCG potency.Clin Exp Immunol. 2015 Aug;181(2):286-96. doi: 10.1111/cei.12634. Epub 2015 Jun 3. Clin Exp Immunol. 2015. PMID: 25845290 Free PMC article.
-
Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein.Clin Dev Immunol. 2011;2011:617892. doi: 10.1155/2011/617892. Epub 2011 Feb 27. Clin Dev Immunol. 2011. PMID: 21461375 Free PMC article.
-
Using a prime and pull approach, lentivector vaccines expressing Ag85A induce immunogenicity but fail to induce protection against Mycobacterium bovis bacillus Calmette-Guérin challenge in mice.Immunology. 2015 Oct;146(2):264-70. doi: 10.1111/imm.12498. Epub 2015 Aug 18. Immunology. 2015. PMID: 26095282 Free PMC article.
-
Multi-stage subunit vaccines against Mycobacterium tuberculosis: an alternative to the BCG vaccine or a BCG-prime boost?Expert Rev Vaccines. 2018 Jan;17(1):31-44. doi: 10.1080/14760584.2018.1406309. Epub 2017 Nov 22. Expert Rev Vaccines. 2018. PMID: 29148853 Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Evaluation of combined BCG and SARS-CoV-2 vaccination for immune enhancement and lung protection in Syrian hamsters.Sci Rep. 2025 May 29;15(1):18908. doi: 10.1038/s41598-025-04246-3. Sci Rep. 2025. PMID: 40442229 Free PMC article.
-
Intravenous BCG vaccination reduces SARS-CoV-2 severity and promotes extensive reprogramming of lung immune cells.iScience. 2023 Aug 24;26(10):107733. doi: 10.1016/j.isci.2023.107733. eCollection 2023 Oct 20. iScience. 2023. PMID: 37674985 Free PMC article.
-
The role of trained immunity in COVID-19: Lessons for the next pandemic.Cell Host Microbe. 2023 Jun 14;31(6):890-901. doi: 10.1016/j.chom.2023.05.004. Cell Host Microbe. 2023. PMID: 37321172 Free PMC article. Review.
-
Dynamic single-cell RNA sequencing reveals BCG vaccination curtails SARS-CoV-2 induced disease severity and lung inflammation.bioRxiv [Preprint]. 2022 Mar 15:2022.03.15.484018. doi: 10.1101/2022.03.15.484018. bioRxiv. 2022. PMID: 35313583 Free PMC article. Preprint.
-
Early introduction of IL-10 weakens BCG revaccination's protection by suppressing CD4+Th1 cell responses.J Transl Med. 2024 Dec 4;22(1):1103. doi: 10.1186/s12967-024-05683-w. J Transl Med. 2024. PMID: 39633471 Free PMC article.
References
-
- WHO Global Tuberculosis Report 2017. Geneva: United Nations, World Health Organization (WHO) (2017). Available online at: http://www.who.int/tb/publications/global_report/en/
-
- WHO . Guidelines on the Management of Latent Tuberculosis Infection. Geneva: World Health Organization; (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

