Sensitive and rapid detection of Zika virus by loop-mediated isothermal amplification
- PMID: 30426316
- PMCID: PMC7089109
- DOI: 10.1007/s11262-018-1612-x
Sensitive and rapid detection of Zika virus by loop-mediated isothermal amplification
Abstract
Zika virus (ZIKV) is a mosquito-borne flavivirus, which is a pathogen affecting humans in Africa, Asia, and America. It is necessary to detect ZIKV with a rapid and sensitive molecular method to guide timely treatment. In this study, a loop-mediated isothermal amplification (LAMP) assay was described, which is an attractive option as a fast, sensitive, and specific method for ZIKV detection using the NS5 protein coding region and the envelope protein (EP) coding region as target sequences. Two different techniques, a calcein/Mn2+ complex chromogenic method and real-time turbidity monitoring, were employed. The specificity and sensitivity of the LAMP assay were determined. The assay's detection limit was 0.5 × 10-9 pmol/µl DNA for NS5 protein coding region and 1.12 × 10-11 pmol/µl DNA for E coding region, respectively, which is a 100-fold increase in sensitivity compared with real-time reverse transcription-polymerase chain reaction (RT-PCR) and conventional PCR. All 12 non-ZIKA respiratory pathogens tested were negative for LAMP detection, indicating the high specificity of the primers for ZIKV. In conclusion, a visual detection LAMP assay was developed, which could be a useful tool for primary quarantine purposes and clinical screening, especially in situations where resources are poor and in point-of-care tests.
Keywords: LAMP; Rapid detection; Sensitivity; Specificity; Zika virus.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures

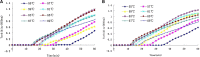

Similar articles
-
Loop-Mediated Isothermal Amplification (LAMP) for the Diagnosis of Zika Virus: A Review.Viruses. 2019 Dec 23;12(1):19. doi: 10.3390/v12010019. Viruses. 2019. PMID: 31877989 Free PMC article. Review.
-
A reverse transcription loop-mediated isothermal amplification for broad coverage detection of Asian and African Zika virus lineages.BMC Infect Dis. 2020 Dec 11;20(1):947. doi: 10.1186/s12879-020-05585-4. BMC Infect Dis. 2020. PMID: 33308203 Free PMC article.
-
Development of Universal and Lineage-Specific Primer Sets for Rapid Detection of the Zika Virus (ZIKV) in Blood and Urine Samples Using One-Step Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP).Jpn J Infect Dis. 2020 Mar 24;73(2):153-156. doi: 10.7883/yoken.JJID.2019.073. Epub 2019 Oct 31. Jpn J Infect Dis. 2020. PMID: 31666491
-
Detection of Zika Virus Using Reverse Transcription-Loop-Mediated Isothermal Amplification (RT-LAMP).Methods Mol Biol. 2020;2142:137-146. doi: 10.1007/978-1-0716-0581-3_12. Methods Mol Biol. 2020. PMID: 32367365
-
Recent progresses and remaining challenges for the detection of Zika virus.Med Res Rev. 2021 Jul;41(4):2039-2108. doi: 10.1002/med.21786. Epub 2021 Feb 9. Med Res Rev. 2021. PMID: 33559917 Review.
Cited by
-
MAT1-1 and MAT1-2 Ophiocordyceps xuefengensis and Comparison of Their Chemical Composition.Biology (Basel). 2024 Sep 2;13(9):686. doi: 10.3390/biology13090686. Biology (Basel). 2024. PMID: 39336113 Free PMC article.
-
Loop-Mediated Isothermal Amplification (LAMP) for the Diagnosis of Zika Virus: A Review.Viruses. 2019 Dec 23;12(1):19. doi: 10.3390/v12010019. Viruses. 2019. PMID: 31877989 Free PMC article. Review.
-
Development and Validation of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) for Rapid Detection of ZIKV in Mosquito Samples from Brazil.Sci Rep. 2019 Mar 14;9(1):4494. doi: 10.1038/s41598-019-40960-5. Sci Rep. 2019. PMID: 30872672 Free PMC article.
-
Development and validation of a one-step reverse transcription loop-mediated isothermal amplification (RT-LAMP) for rapid detection of ZIKV in patient samples from Brazil.Sci Rep. 2021 Feb 18;11(1):4111. doi: 10.1038/s41598-021-83371-1. Sci Rep. 2021. PMID: 33602985 Free PMC article.
-
Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of SARS-CoV-2.PeerJ. 2020 Jun 3;8:e9278. doi: 10.7717/peerj.9278. eCollection 2020. PeerJ. 2020. PMID: 32547882 Free PMC article.
References
-
- Eppes C, Rac M, Dunn J, Versalovic J, Murray KO, Suter MA, Sanz Cortes M, Espinoza J, Seferovic MD, Lee W, Hotez P, Mastrobattista J, Clark SL, Belfort MA, Aagaard KM. Testing for Zika virus (ZIKV) infection in pregnancy: key concepts to deal with an emerging epidemic. Am J Obstet Gynecol. 2017;213(3):209225. doi: 10.1016/j.ajog.2017.01.020. - DOI - PubMed
-
- Sakkas H, Economou V, Papadopoulou C. Zika virus infection: past and present of another emerging vector-borne disease. J Vector Borne Dis. 2016;53:305–311. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

