Serum from Asthmatic Mice Potentiates the Therapeutic Effects of Mesenchymal Stromal Cells in Experimental Allergic Asthma
- PMID: 30426724
- PMCID: PMC6392406
- DOI: 10.1002/sctm.18-0056
Serum from Asthmatic Mice Potentiates the Therapeutic Effects of Mesenchymal Stromal Cells in Experimental Allergic Asthma
Abstract
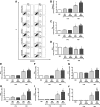
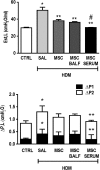
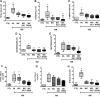
Asthma is a chronic inflammatory disease characterized by airway inflammation and remodeling, which can lead to progressive decline of lung function. Although mesenchymal stromal cells (MSCs) have shown beneficial immunomodulatory properties in preclinical models of allergic asthma, effects on airway remodeling have been limited. Mounting evidence suggests that prior exposure of MSCs to specific inflammatory stimuli or environments can enhance their immunomodulatory properties. Therefore, we investigated whether stimulating MSCs with bronchoalveolar lavage fluid (BALF) or serum from asthmatic mice could potentiate their therapeutic properties in experimental asthma. In a house dust mite (HDM) extract asthma model in mice, unstimulated, asthmatic BALF-stimulated, or asthmatic serum-stimulated MSCs were administered intratracheally 24 hours after the final HDM challenge. Lung mechanics and histology; BALF protein, cellularity, and biomarker levels; and lymph-node and bone marrow cellularity were assessed. Compared with unstimulated or BALF-stimulated MSCs, serum-stimulated MSCs further reduced BALF levels of interleukin (IL)-4, IL-13, and eotaxin, total and differential cellularity in BALF, bone marrow and lymph nodes, and collagen fiber content, while increasing BALF IL-10 levels and improving lung function. Serum stimulation led to higher MSC apoptosis, expression of various mediators (transforming growth factor-β, interferon-γ, IL-10, tumor necrosis factor-α-stimulated gene 6 protein, indoleamine 2,3-dioxygenase-1, and IL-1 receptor antagonist), and polarization of macrophages to M2 phenotype. In conclusion, asthmatic serum may be a novel strategy to potentiate therapeutic effects of MSCs in experimental asthma, leading to further reductions in both inflammation and remodeling than can be achieved with unstimulated MSCs. Stem Cells Translational Medicine 2019;8:301&312.
Keywords: Animal models; Bone marrow stromal cells; Cell therapy; Eosinophils; Lung; Macrophages.
© 2018 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
D.J.W. has received research funding from Athersys Inc., Biotek Inc., and United Therapeutics Inc. The other authors indicated no potential conflicts of interest.
Figures




References
-
- Global Strategy for Asthma Management and Prevention . Global Iniciative for Asthma; 2018. Available at https://ginasthma.org/2018‐gina‐report‐global‐strategy‐for‐asthma‐manage.... Accessed September 27, 2018.
-
- Royce SG, Tang ML. The effects of current therapies on airway remodeling in asthma and new possibilities for treatment and prevention. Curr Mol Pharmacol 2009;2:169–181. - PubMed
-
- Barnes PJ. New therapies for asthma: Is there any progress? Trends Pharmacol Sci 2010;31:335–343. - PubMed
-
- Papierniak ES, Lowenthal DT, Harman E. Novel therapies in asthma: Leukotriene antagonists, biologic agents, and beyond. Am J Ther 2013;20:79–103. - PubMed
-
- Cruz FF, Borg ZD, Goodwin M et al. Systemic administration of human bone marrow‐derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract‐induced allergic airway inflammation in immunocompetent mice. Stem Cells Translational Medicine 2015;4:1302–1316. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

