Building and Regenerating the Lung Cell by Cell
- PMID: 30427276
- PMCID: PMC6442926
- DOI: 10.1152/physrev.00001.2018
Building and Regenerating the Lung Cell by Cell
Abstract
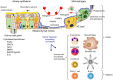
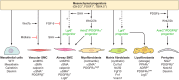
The unique architecture of the mammalian lung is required for adaptation to air breathing at birth and thereafter. Understanding the cellular and molecular mechanisms controlling its morphogenesis provides the framework for understanding the pathogenesis of acute and chronic lung diseases. Recent single-cell RNA sequencing data and high-resolution imaging identify the remarkable heterogeneity of pulmonary cell types and provides cell selective gene expression underlying lung development. We will address fundamental issues related to the diversity of pulmonary cells, to the formation and function of the mammalian lung, and will review recent advances regarding the cellular and molecular pathways involved in lung organogenesis. What cells form the lung in the early embryo? How are cell proliferation, migration, and differentiation regulated during lung morphogenesis? How do cells interact during lung formation and repair? How do signaling and transcriptional programs determine cell-cell interactions necessary for lung morphogenesis and function?
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



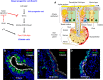
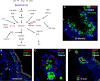
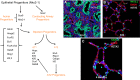

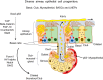



References
-
- Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. doi:10.1152/ajplung.00050.2006. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

