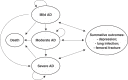
Cost-effectiveness analysis of the treatment of mild and moderate Alzheimer's disease in Brazil
- PMID: 30427385
- PMCID: PMC6794128
- DOI: 10.1590/1516-4446-2017-0021
Cost-effectiveness analysis of the treatment of mild and moderate Alzheimer's disease in Brazil
Abstract
Objective: To perform a cost-effectiveness analysis of donepezil and rivastigmine therapy for mild and moderate Alzheimer's disease (AD) from the perspective of the Brazilian Unified Health System.
Method: A hypothetical cohort of 1,000 individuals of both sexes, aged >65 years, and diagnosed with AD was simulated using a Markov model. The time horizon was 10 years, with 1-year cycles. A deterministic and probabilistic sensitivity analysis was performed.
Results: For mild AD, the study showed an increase in quality-adjusted life years (QALYs) of 0.61 QALY/21,907.38 Brazilian reais (BRL) for patients treated with donepezil and 0.58 QALY/BRL 24,683.33 for patients treated with rivastigmine. In the moderate AD group, QALY increases of 0.05/BRL 27,414.96 were observed for patients treated with donepezil and 0.06/BRL 34,222.96 for patients treated with rivastigmine.
Conclusions: The findings of this study contradict the standard of care for mild and moderate AD in Brazil, which is based on rivastigmine. A pharmacological treatment option based on current Brazilian clinical practice guidelines for AD suggests that rivastigmine is less cost-effective (0.39 QALY/BRL 32,685.77) than donepezil. Probabilistic analysis indicates that donepezil is the most cost-effective treatment for mild and moderate AD.
Conflict of interest statement
VM has received grants from Novartis, Wyeth, EMS, Janssen-Cilag, Apsen Farmacê utica, Aché, Lundbeck, Lilly, moksha8, and Servier. JL has received grants from Novartis, Wyeth, EMS, Janssen-Cilag, Apsen Farmacêutica, Aché, Lundbeck, Lilly, moksha8, and Servier. The other authors report no conflicts of interest.
Figures
Similar articles
-
Donepezil and rivastigmine in the treatment of Alzheimer's disease: a best-evidence synthesis of the published data on their efficacy and cost-effectiveness.Clin Ther. 2002 Jun;24(6):862-86; discussion 837. doi: 10.1016/s0149-2918(02)80004-2. Clin Ther. 2002. PMID: 12117079 Review.
-
Savings in the cost of caring for patients with Alzheimer's disease in Canada: an analysis of treatment with rivastigmine.Clin Ther. 2000 Apr;22(4):439-51. doi: 10.1016/s0149-2918(00)89012-8. Clin Ther. 2000. PMID: 10823365 Clinical Trial.
-
Comparison of cholinesterase inhibitor utilization patterns and associated health care costs in Alzheimer's disease.J Manag Care Pharm. 2008 Jun;14(5):451-61. doi: 10.18553/jmcp.2008.14.5.451. J Manag Care Pharm. 2008. PMID: 18597574 Free PMC article.
-
Pharmaceutical Treatment for Alzheimer's Disease and Related Dementias: Utilization and Disparities.J Alzheimers Dis. 2020;76(2):579-589. doi: 10.3233/JAD-200133. J Alzheimers Dis. 2020. PMID: 32538845 Free PMC article.
-
[Anti-Dementia Drugs (Anti-Alzheimer's Disease Drugs)].Brain Nerve. 2023 May;75(5):464-469. doi: 10.11477/mf.1416202360. Brain Nerve. 2023. PMID: 37194514 Review. Japanese.
Cited by
-
Alzheimer's disease: an epidemiological analysis over the number of hospitalizations and deaths in Brazil.Arq Neuropsiquiatr. 2023 Jun;81(6):577-584. doi: 10.1055/s-0043-1767827. Epub 2023 Jun 28. Arq Neuropsiquiatr. 2023. PMID: 37379869 Free PMC article.
-
Model-Based Economic Evaluations of Interventions for Dementia: An Updated Systematic Review and Quality Assessment.Appl Health Econ Health Policy. 2024 Jul;22(4):503-525. doi: 10.1007/s40258-024-00878-0. Epub 2024 Mar 30. Appl Health Econ Health Policy. 2024. PMID: 38554246 Free PMC article.
References
-
- World Health Organization/Alzheimer’s Disease International (WHO/ADI) Dementia: a public health priority, [Internet] 2012. [cited 2018 May 23] www.who.int/mental_health/publications/dementia_report_2012/en/
-
- Bottino CM, Laks J, Blay SL. Demência e transtornos cognitivos em idosos. In: Machado JCB, editor. Diagnóstico clínico na doença de Alzheimer. Rio de Janeiro: Guanabara Koogan; 2006. pp. 173–6.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical