Genetic ablation or pharmacological inhibition of Kv1.1 potassium channel subunits impairs atrial repolarization in mice
- PMID: 30427720
- PMCID: PMC6397341
- DOI: 10.1152/ajpcell.00335.2018
Genetic ablation or pharmacological inhibition of Kv1.1 potassium channel subunits impairs atrial repolarization in mice
Abstract
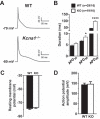
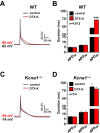
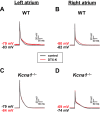
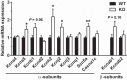
Voltage-gated Kv1.1 potassium channel α-subunits, encoded by the Kcna1 gene, have traditionally been regarded as neural-specific with no expression or function in the heart. However, recent data revealed that Kv1.1 subunits are expressed in atria where they may have an overlooked role in controlling repolarization and arrhythmia susceptibility independent of the nervous system. To explore this concept in more detail and to identify functional and molecular effects of Kv1.1 channel impairment in the heart, atrial cardiomyocyte patch-clamp electrophysiology and gene expression analyses were performed using Kcna1 knockout ( Kcna1-/-) mice. Specifically, we hypothesized that Kv1.1 subunits contribute to outward repolarizing K+ currents in mouse atria and that their absence prolongs cardiac action potentials. In voltage-clamp experiments, dendrotoxin-K (DTX-K), a Kv1.1-specific inhibitor, significantly reduced peak outward K+ currents in wild-type (WT) atrial cells but not Kcna1-/- cells, demonstrating an important contribution by Kv1.1-containing channels to mouse atrial repolarizing currents. In current-clamp recordings, Kcna1-/- atrial myocytes exhibited significant action potential prolongation which was exacerbated in right atria, effects that were partially recapitulated in WT cells by application of DTX-K. Quantitative RT-PCR measurements showed mRNA expression remodeling in Kcna1-/- atria for several ion channel genes that contribute to the atrial action potential including the Kcna5, Kcnh2, and Kcnj2 potassium channel genes and the Scn5a sodium channel gene. This study demonstrates a previously undescribed heart-intrinsic role for Kv1.1 subunits in mediating atrial repolarization, thereby adding a new member to the already diverse collection of known K+ channels in the heart.
Keywords: Kv1.1; action potential; atria; channel remodeling; dendrotoxin-K.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Epilepsy-associated Kv1.1 channel subunits regulate intrinsic cardiac pacemaking in mice.J Gen Physiol. 2024 Sep 2;156(9):e202413578. doi: 10.1085/jgp.202413578. Epub 2024 Jul 22. J Gen Physiol. 2024. PMID: 39037413 Free PMC article.
-
Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation.Basic Res Cardiol. 2015 Sep;110(5):505. doi: 10.1007/s00395-015-0505-6. Epub 2015 Jul 11. Basic Res Cardiol. 2015. PMID: 26162324 Free PMC article.
-
Kv1.1 potassium channel subunit deficiency alters ventricular arrhythmia susceptibility, contractility, and repolarization.Physiol Rep. 2021 Jan;9(1):e14702. doi: 10.14814/phy2.14702. Physiol Rep. 2021. PMID: 33427415 Free PMC article.
-
Kv1.1 channel subunits in the control of neurocardiac function.Channels (Austin). 2019 Dec;13(1):299-307. doi: 10.1080/19336950.2019.1635864. Channels (Austin). 2019. PMID: 31250689 Free PMC article. Review.
-
Atrial-Selective Potassium Channel Blockers.Card Electrophysiol Clin. 2016 Jun;8(2):411-21. doi: 10.1016/j.ccep.2016.02.005. Epub 2016 Mar 24. Card Electrophysiol Clin. 2016. PMID: 27261831 Review.
Cited by
-
Kv1.1 Channelopathies: Pathophysiological Mechanisms and Therapeutic Approaches.Int J Mol Sci. 2020 Apr 22;21(8):2935. doi: 10.3390/ijms21082935. Int J Mol Sci. 2020. PMID: 32331416 Free PMC article. Review.
-
Cardiac-specific Kv1.1 deficiency alters cardiomyocyte electrophysiology without modifying overall cardiac function or arrhythmia susceptibility.bioRxiv [Preprint]. 2025 Aug 28:2025.08.25.671830. doi: 10.1101/2025.08.25.671830. bioRxiv. 2025. PMID: 40909726 Free PMC article. Preprint.
-
Functional study of a KCNH2 mutant: Novel insights on the pathogenesis of the LQT2 syndrome.J Cell Mol Med. 2019 Sep;23(9):6331-6342. doi: 10.1111/jcmm.14521. Epub 2019 Jul 30. J Cell Mol Med. 2019. PMID: 31361068 Free PMC article.
-
Epilepsy-associated Kv1.1 channel subunits regulate intrinsic cardiac pacemaking in mice.J Gen Physiol. 2024 Sep 2;156(9):e202413578. doi: 10.1085/jgp.202413578. Epub 2024 Jul 22. J Gen Physiol. 2024. PMID: 39037413 Free PMC article.
-
Kv1.1 channels help set the pace.J Gen Physiol. 2024 Sep 2;156(9):e202413649. doi: 10.1085/jgp.202413649. Epub 2024 Aug 7. J Gen Physiol. 2024. PMID: 39110119 Free PMC article.
References
-
- Biet M, Morin N, Lessard-Beaudoin M, Graham RK, Duss S, Gagné J, Sanon NT, Carmant L, Dumaine R. Prolongation of action potential duration and QT interval during epilepsy linked to increased contribution of neuronal sodium channels to cardiac late Na+ current: potential mechanism for sudden death in epilepsy. Circ Arrhythm Electrophysiol 8: 912–920, 2015. doi:10.1161/CIRCEP.114.002693. - DOI - PubMed
-
- Glasscock E, Voigt N, McCauley MD, Sun Q, Li N, Chiang DY, Zhou XB, Molina CE, Thomas D, Schmidt C, Skapura DG, Noebels JL, Dobrev D, Wehrens XH. Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation. Basic Res Cardiol 110: 505, 2015. doi:10.1007/s00395-015-0505-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

