Automated high-throughput light-sheet fluorescence microscopy of larval zebrafish
- PMID: 30427839
- PMCID: PMC6235235
- DOI: 10.1371/journal.pone.0198705
Automated high-throughput light-sheet fluorescence microscopy of larval zebrafish
Abstract
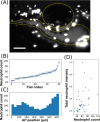
Light sheet fluorescence microscopy enables fast, minimally phototoxic, three-dimensional imaging of live specimens, but is currently limited by low throughput and tedious sample preparation. Here, we describe an automated high-throughput light sheet fluorescence microscope in which specimens are positioned by and imaged within a fluidic system integrated with the sheet excitation and detection optics. We demonstrate the ability of the instrument to rapidly examine live specimens with minimal manual intervention by imaging fluorescent neutrophils over a nearly 0.3 mm3 volume in dozens of larval zebrafish. In addition to revealing considerable inter-individual variability in neutrophil number, known previously from labor-intensive methods, three-dimensional imaging allows assessment of the correlation between the bulk measure of total cellular fluorescence and the spatially resolved measure of actual neutrophil number per animal. We suggest that our simple experimental design should considerably expand the scope and impact of light sheet imaging in the life sciences.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Keller PJ, Ahrens MB, Freeman J. Light-sheet imaging for systems neuroscience [Internet]. Nature Methods. 2014. [cited 2018 Mar 22]. Available from: https://www.nature.com/articles/nmeth.3214 - PubMed
-
- Huisken J. Slicing embryos gently with laser light sheets. BioEssays [Internet]. 2012. [cited 2018 Mar 22];34 Available from: https://www.readcube.com/articles/10.1002/bies.201100120 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

