DNA-PK Is Targeted by Multiple Vaccinia Virus Proteins to Inhibit DNA Sensing
- PMID: 30428360
- PMCID: PMC6250978
- DOI: 10.1016/j.celrep.2018.10.034
DNA-PK Is Targeted by Multiple Vaccinia Virus Proteins to Inhibit DNA Sensing
Abstract
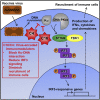
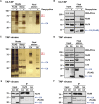
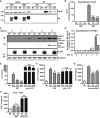
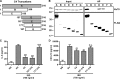
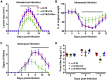
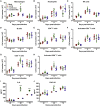
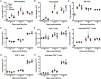
Virus infection is sensed by pattern recognition receptors (PRRs) detecting virus nucleic acids and initiating an innate immune response. DNA-dependent protein kinase (DNA-PK) is a PRR that binds cytosolic DNA and is antagonized by vaccinia virus (VACV) protein C16. Here, VACV protein C4 is also shown to antagonize DNA-PK by binding to Ku and blocking Ku binding to DNA, leading to a reduced production of cytokines and chemokines in vivo and a diminished recruitment of inflammatory cells. C4 and C16 share redundancy in that a double deletion virus has reduced virulence not seen with single deletion viruses following intradermal infection. However, non-redundant functions exist because both single deletion viruses display attenuated virulence compared to wild-type VACV after intranasal infection. It is notable that VACV expresses two proteins to antagonize DNA-PK, but it is not known to target other DNA sensors, emphasizing the importance of this PRR in the response to infection in vivo.
Keywords: DNA protein kinase; DNA sensing; IRF3 signaling; immune evasion; pattern recognition receptor; protein C4; vaccinia virus; virulence factor.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Ahn J., Barber G.N. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Curr. Opin. Immunol. 2014;31:121–126. - PubMed
-
- Alcamí A., Smith G.L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. - PubMed
-
- Bartlett N., Symons J.A., Tscharke D.C., Smith G.L. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J. Gen. Virol. 2002;83:1965–1976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

