N-Heterocyclic-Carbene-Catalyzed Domino Reactions via Two or More Activation Modes
- PMID: 30428366
- PMCID: PMC6135926
- DOI: 10.1016/j.isci.2018.03.006
N-Heterocyclic-Carbene-Catalyzed Domino Reactions via Two or More Activation Modes
Abstract
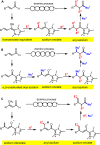
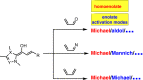
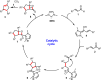
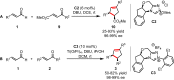
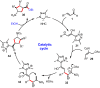
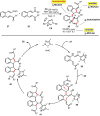
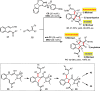
Organocatalytic domino processes have become a rapidly growing area of research. N-heterocyclic carbenes (NHCs) have emerged as powerful organocatalysts for various transformations and continue to have widespread application. In the last decade, domino reactions catalyzed by NHCs have seen significant progress since the different activation modes could be successfully combined in one process. The most attractive features of these domino sequences include the readily available catalysts and substrates, the simple operational procedures, and the rapid assembly of complex molecular scaffolds with excellent levels of stereocontrol under mild reaction conditions. This review covers the advances in NHC-catalyzed domino reactions by focusing on the reaction scope, limitations, and mechanism with a close attention to the features of the reaction substrates.
Keywords: Organic Chemistry Methods; Organic Synthesis; Stereochemistry.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




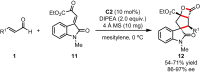

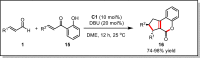



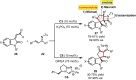
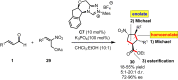
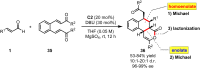











References
-
- Ahrendt K.A., Borths C.J., MacMillan D.W.C. New strategies for organic catalysis: the first highly enantioselective organocatalytic Diels−Alder reaction. J. Am. Chem. Soc. 2000;122:4243–4244.
-
- Albrecht Ł., Jiang H., Jørgensen K.A. A simple recipe for sophisticated cocktails: organocatalytic one-pot reactions-concept, nomenclature, and future perspectives. Angew. Chem. Int. Ed. 2011;50:8492–8509. - PubMed
-
- Bera S., Daniliuc C.G., Studer A. Enantioselective synthesis of substituted δ-lactones by cooperative oxidative N-heterocyclic carbene and Lewis acid catalysis. Org. Lett. 2015;17:4940–4943. - PubMed
-
- Bera S., Samanta R.C., Daniliuc C.G., Studer A. Asymmetric synthesis of highly substituted β-lactones through oxidative carbene catalysis with LiCl as cooperative Lewis acid. Angew. Chem. Int. Ed. 2014;53:9622–9626. - PubMed
-
- Bhunia A., Patra A., Puranik V.G., Biju A.T. NHC-catalyzed reaction of enals with hydroxy chalcones: diastereoselective synthesis of functionalized coumarins. Org. Lett. 2013;15:1756–1759. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

