Neuroprotection Comparison of Rosmarinic Acid and Carnosic Acid in Primary Cultures of Cerebellar Granule Neurons
- PMID: 30428519
- PMCID: PMC6278428
- DOI: 10.3390/molecules23112956
Neuroprotection Comparison of Rosmarinic Acid and Carnosic Acid in Primary Cultures of Cerebellar Granule Neurons
Abstract
Neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS), Alzheimer's disease, and Parkinson's disease, are characterized by the progressive loss of neurons in specific regions of the brain and/or spinal cord. Neuronal cell loss typically occurs by either apoptotic or necrotic mechanisms. Oxidative stress and nitrosative stress, along with excitotoxicity and caspase activation, have all been implicated as major underlying causes of neuronal cell death. Diverse nutraceuticals (bioactive compounds found in common foods) have been shown to have neuroprotective effects in a variety of in vitro and in vivo disease models. In the current study, we compared the neuroprotective effects of two polyphenolic compounds, rosmarinic acid and carnosic acid, which are both found at substantial concentrations in the herb rosemary. The capacity of these compounds to rescue primary cultures of rat cerebellar granule neurons (CGNs) from a variety of stressors was investigated. Both polyphenols significantly reduced CGN death induced by the nitric oxide donor, sodium nitroprusside (nitrosative stress). Rosmarinic acid uniquely protected CGNs from glutamate-induced excitotoxicity, while only carnosic acid rescued CGNs from caspase-dependent apoptosis induced by removal of depolarizing extracellular potassium (5K apoptotic condition). Finally, we found that carnosic acid protects CGNs from 5K-induced apoptosis by activating a phosphatidylinositol 3-kinase (PI3K) pro-survival pathway. The shared and unique neuroprotective effects of these two compounds against diverse modes of neuronal cell death suggest that future preclinical studies should explore the potential complementary effects of these rosemary polyphenols on neurodegenerative disease progression.
Keywords: antioxidants; neuronal apoptosis; nutraceuticals; polyphenols; rosemary.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



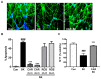
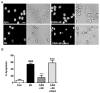

Similar articles
-
Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons.Brain Res. 2016 Oct 1;1648(Pt A):69-80. doi: 10.1016/j.brainres.2016.07.028. Epub 2016 Jul 18. Brain Res. 2016. PMID: 27444557
-
Cr (VI) induced oxidative stress and toxicity in cultured cerebellar granule neurons at different stages of development and protective effect of Rosmarinic acid.Environ Toxicol. 2016 Mar;31(3):269-77. doi: 10.1002/tox.22041. Epub 2014 Sep 12. Environ Toxicol. 2016. PMID: 25213303
-
Differential activation of pregnane X receptor by carnosic acid, carnosol, ursolic acid, and rosmarinic acid.Pharmacol Res. 2017 Jun;120:23-33. doi: 10.1016/j.phrs.2017.03.007. Epub 2017 Mar 10. Pharmacol Res. 2017. PMID: 28288941
-
Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer's and Vascular Dementia Drugs.Int J Mol Sci. 2018 Feb 3;19(2):458. doi: 10.3390/ijms19020458. Int J Mol Sci. 2018. PMID: 29401682 Free PMC article. Review.
-
Nutraceutical antioxidants as novel neuroprotective agents.Molecules. 2010 Nov 3;15(11):7792-814. doi: 10.3390/molecules15117792. Molecules. 2010. PMID: 21060289 Free PMC article. Review.
Cited by
-
A Validated Method for the Determination of Carnosic Acid and Carnosol in the Fresh Foliage of Salvia rosmarinus and Salvia officinalis from Greece.Plants (Basel). 2022 Nov 15;11(22):3106. doi: 10.3390/plants11223106. Plants (Basel). 2022. PMID: 36432835 Free PMC article.
-
Neuroprotective Effect of Sterculia setigera Leaves Hydroethanolic Extract.J Mol Neurosci. 2024 Apr 17;74(2):44. doi: 10.1007/s12031-024-02222-6. J Mol Neurosci. 2024. PMID: 38630337
-
Metal Chelation Therapy and Parkinson's Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs.Biomolecules. 2019 Jul 9;9(7):269. doi: 10.3390/biom9070269. Biomolecules. 2019. PMID: 31324037 Free PMC article. Review.
-
The Effect of Rosmarinic Acid on Neural Differentiation of Wartons Jelly-derived Mesenchymal Stem Cells in Two-dimensional and Three-dimensional Cultures using Chitosan-based Hydrogel.Basic Clin Neurosci. 2023 Jan-Feb;14(1):117-128. doi: 10.32598/bcn.2021.2596.1. Epub 2023 Jan 1. Basic Clin Neurosci. 2023. PMID: 37346869 Free PMC article.
-
Rosmarinic Acid Reduces Microglia Senescence: A Novel Therapeutic Approach for the Management of Neuropathic Pain Symptoms.Biomedicines. 2022 Jun 21;10(7):1468. doi: 10.3390/biomedicines10071468. Biomedicines. 2022. PMID: 35884774 Free PMC article.
References
-
- Rajasekaran A., Sivagnanam G., Xavier R. Nutraceuticals as therapeutic agents: A review. Res. J. Pharm. Technol. 2008;1:171–174.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

