Characterization of TrxC, an Atypical Thioredoxin Exclusively Present in Cyanobacteria
- PMID: 30428557
- PMCID: PMC6262485
- DOI: 10.3390/antiox7110164
Characterization of TrxC, an Atypical Thioredoxin Exclusively Present in Cyanobacteria
Abstract
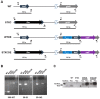
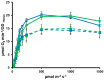
Cyanobacteria form a diverse group of oxygenic photosynthetic prokaryotes considered to be the antecessor of plant chloroplast. They contain four different thioredoxins isoforms, three of them corresponding to m, x and y type present in plant chloroplast, while the fourth one (named TrxC) is exclusively found in cyanobacteria. TrxC has a modified active site (WCGLC) instead of the canonical (WCGPC) present in most thioredoxins. We have purified it and assayed its activity but surprisingly TrxC lacked all the classical activities, such as insulin precipitation or activation of the fructose-1,6-bisphosphatase. Mutants lacking trxC or over-expressing it were generated in the model cyanobacterium Synechocystis sp. PCC 6803 and their phenotypes have been analyzed. The ΔtrxC mutant grew at similar rates to WT in all conditions tested although it showed an increased carotenoid content especially under low carbon conditions. Overexpression strains showed reduced growth under the same conditions and accumulated lower amounts of carotenoids. They also showed lower oxygen evolution rates at high light but higher Fv'/Fm' and Non-photochemical-quenching (NPQ) in dark adapted cells, suggesting a more oxidized plastoquinone pool. All these data suggest that TrxC might have a role in regulating photosynthetic adaptation to low carbon and/or high light conditions.
Keywords: cyanobacteria; photosynthesis; thioredoxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Contrasting modes of photosynthetic enzyme regulation in oxygenic and anoxygenic prokaryotes.Arch Microbiol. 1984 Oct;139(2-3):124-9. doi: 10.1007/BF00401986. Arch Microbiol. 1984. PMID: 11536590
-
Photosynthetic regulation of the cyanobacterium Synechocystis sp. PCC 6803 thioredoxin system and functional analysis of TrxB (Trx x) and TrxQ (Trx y) thioredoxins.Mol Plant. 2009 Mar;2(2):270-83. doi: 10.1093/mp/ssn070. Epub 2008 Nov 17. Mol Plant. 2009. PMID: 19825613
-
Photosystem activity and state transitions of the photosynthetic apparatus in cyanobacterium Synechocystis PCC 6803 mutants with different redox state of the plastoquinone pool.Biochemistry (Mosc). 2015 Jan;80(1):50-60. doi: 10.1134/S000629791501006X. Biochemistry (Mosc). 2015. PMID: 25754039
-
Disulphide proteomes and interactions with thioredoxin on the track towards understanding redox regulation in chloroplasts and cyanobacteria.J Proteomics. 2009 Apr 13;72(3):416-38. doi: 10.1016/j.jprot.2009.01.003. Epub 2009 Jan 13. J Proteomics. 2009. PMID: 19185068 Review.
-
Carotenoids, versatile components of oxygenic photosynthesis.Prog Lipid Res. 2013 Oct;52(4):539-61. doi: 10.1016/j.plipres.2013.07.001. Epub 2013 Jul 26. Prog Lipid Res. 2013. PMID: 23896007 Review.
Cited by
-
Depletion of m-type thioredoxin impairs photosynthesis, carbon fixation, and oxidative stress in cyanobacteria.Plant Physiol. 2021 Nov 3;187(3):1325-1340. doi: 10.1093/plphys/kiab321. Plant Physiol. 2021. PMID: 34618018 Free PMC article.
-
Thioredoxin and Glutaredoxin Systems Antioxidants Special Issue.Antioxidants (Basel). 2019 Mar 18;8(3):68. doi: 10.3390/antiox8030068. Antioxidants (Basel). 2019. PMID: 30889816 Free PMC article.
-
Thioredoxin A regulates protein synthesis to maintain carbon and nitrogen partitioning in cyanobacteria.Plant Physiol. 2024 Jul 31;195(4):2921-2936. doi: 10.1093/plphys/kiae101. Plant Physiol. 2024. PMID: 38386687 Free PMC article.
-
Exploring the Diversity of the Thioredoxin Systems in Cyanobacteria.Antioxidants (Basel). 2022 Mar 28;11(4):654. doi: 10.3390/antiox11040654. Antioxidants (Basel). 2022. PMID: 35453339 Free PMC article. Review.
-
Association of Acidotolerant Cyanobacteria to Microbial Mats below pH 1 in Acidic Mineral Precipitates in Río Tinto River in Spain.Microorganisms. 2024 Apr 19;12(4):829. doi: 10.3390/microorganisms12040829. Microorganisms. 2024. PMID: 38674771 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

