Crystallographic and Computational Characterization of Methyl Tetrel Bonding in S-Adenosylmethionine-Dependent Methyltransferases
- PMID: 30428636
- PMCID: PMC6278250
- DOI: 10.3390/molecules23112965
Crystallographic and Computational Characterization of Methyl Tetrel Bonding in S-Adenosylmethionine-Dependent Methyltransferases
Abstract
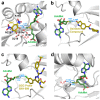
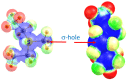
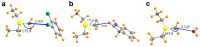
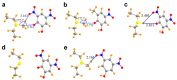
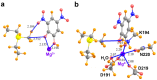
Tetrel bonds represent a category of non-bonding interaction wherein an electronegative atom donates a lone pair of electrons into the sigma antibonding orbital of an atom in the carbon group of the periodic table. Prior computational studies have implicated tetrel bonding in the stabilization of a preliminary state that precedes the transition state in SN2 reactions, including methyl transfer. Notably, the angles between the tetrel bond donor and acceptor atoms coincide with the prerequisite geometry for the SN2 reaction. Prompted by these findings, we surveyed crystal structures of methyltransferases in the Protein Data Bank and discovered multiple instances of carbon tetrel bonding between the methyl group of the substrate S-adenosylmethionine (AdoMet) and electronegative atoms of small molecule inhibitors, ions, and solvent molecules. The majority of these interactions involve oxygen atoms as the Lewis base, with the exception of one structure in which a chlorine atom of an inhibitor functions as the electron donor. Quantum mechanical analyses of a representative subset of the methyltransferase structures from the survey revealed that the calculated interaction energies and spectral properties are consistent with the values for bona fide carbon tetrel bonds. The discovery of methyl tetrel bonding offers new insights into the mechanism underlying the SN2 reaction catalyzed by AdoMet-dependent methyltransferases. These findings highlight the potential of exploiting these interactions in developing new methyltransferase inhibitors.
Keywords: AdoMet; S-adenosylmethionine; SAM; SN2 reaction; charge transfer; methyl transfer; methylation; methyltransferase; molecular electrostatic potential; noncovalent bond; sigma-hole; tetrel bond.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Conservation and functional importance of carbon-oxygen hydrogen bonding in AdoMet-dependent methyltransferases.J Am Chem Soc. 2013 Oct 16;135(41):15536-48. doi: 10.1021/ja407140k. Epub 2013 Oct 7. J Am Chem Soc. 2013. PMID: 24093804
-
Sulfur-Oxygen Chalcogen Bonding Mediates AdoMet Recognition in the Lysine Methyltransferase SET7/9.ACS Chem Biol. 2016 Mar 18;11(3):748-54. doi: 10.1021/acschembio.5b00852. Epub 2016 Jan 12. ACS Chem Biol. 2016. PMID: 26713889
-
Strong Tetrel Bonds: Theoretical Aspects and Experimental Evidence.Molecules. 2018 Oct 15;23(10):2642. doi: 10.3390/molecules23102642. Molecules. 2018. PMID: 30326582 Free PMC article.
-
Hydrogen Bond and Other Lewis Acid-Lewis Base Interactions as Preliminary Stages of Chemical Reactions.Molecules. 2020 Oct 13;25(20):4668. doi: 10.3390/molecules25204668. Molecules. 2020. PMID: 33066201 Free PMC article. Review.
-
Methyltransferase Inhibitors: Competing with, or Exploiting the Bound Cofactor.Molecules. 2019 Dec 8;24(24):4492. doi: 10.3390/molecules24244492. Molecules. 2019. PMID: 31817960 Free PMC article. Review.
Cited by
-
Taxonomy of Chemical Bondings: Opportunities and Challenges.Angew Chem Int Ed Engl. 2025 Jul;64(27):e202506525. doi: 10.1002/anie.202506525. Epub 2025 May 22. Angew Chem Int Ed Engl. 2025. PMID: 40401347 Free PMC article.
-
Theoretical Studies of IR and NMR Spectral Changes Induced by Sigma-Hole Hydrogen, Halogen, Chalcogen, Pnicogen, and Tetrel Bonds in a Model Protein Environment.Molecules. 2019 Sep 12;24(18):3329. doi: 10.3390/molecules24183329. Molecules. 2019. PMID: 31547416 Free PMC article.
-
Dual Geometry Schemes in Tetrel Bonds: Complexes between TF₄ (T = Si, Ge, Sn) and Pyridine Derivatives.Molecules. 2019 Jan 21;24(2):376. doi: 10.3390/molecules24020376. Molecules. 2019. PMID: 30669688 Free PMC article.
-
The Realm of Unconventional Noncovalent Interactions in Proteins: Their Significance in Structure and Function.ACS Omega. 2023 Jun 13;8(25):22268-22284. doi: 10.1021/acsomega.3c00205. eCollection 2023 Jun 27. ACS Omega. 2023. PMID: 37396257 Free PMC article. Review.
-
Induction of Innate Immune Memory by Engineered Nanoparticles in Monocytes/Macrophages: From Hypothesis to Reality.Front Immunol. 2020 Oct 6;11:566309. doi: 10.3389/fimmu.2020.566309. eCollection 2020. Front Immunol. 2020. PMID: 33123137 Free PMC article. Review.
References
-
- Woodard R.W., Tsai M.D., Floss H.G., Crooks P.A., Coward J.K. Stereochemical course of the transmethylation catalyzed by catechol O-methyltransferase. J. Biol. Chem. 1980;255:9124–9127. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

