Interfacing Pathogen Detection with Smartphones for Point-of-Care Applications
- PMID: 30428666
- PMCID: PMC6867037
- DOI: 10.1021/acs.analchem.8b04973
Interfacing Pathogen Detection with Smartphones for Point-of-Care Applications
Abstract
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Use of the rapid BinaxNOW malaria test in a 24-hour laboratory associated with accurate detection and decreased malaria testing turnaround times in a pediatric setting where malaria is not endemic.J Clin Microbiol. 2013 May;51(5):1567-9. doi: 10.1128/JCM.00293-13. Epub 2013 Feb 13. J Clin Microbiol. 2013. PMID: 23408691 Free PMC article.
-
Aptamers: The Powerful Molecular Tools for Virus Detection.Chem Asian J. 2021 Jun 1;16(11):1298-1306. doi: 10.1002/asia.202100242. Epub 2021 May 1. Chem Asian J. 2021. PMID: 33851522 Review.
-
A module of the DnaJ heat shock proteins found in malaria parasites.Trends Biochem Sci. 1992 Apr;17(4):129. doi: 10.1016/0968-0004(92)90319-5. Trends Biochem Sci. 1992. PMID: 1585456 No abstract available.
-
A Review of Membrane-Based Biosensors for Pathogen Detection.Sensors (Basel). 2015 Jun 15;15(6):14045-78. doi: 10.3390/s150614045. Sensors (Basel). 2015. PMID: 26083229 Free PMC article. Review.
-
Trial with ParaSight-F in the detection of Plasmodium falciparum infection in Chennai (Tamil Nadu), India.Indian J Malariol. 1998 Sep;35(3):160-2. Indian J Malariol. 1998. PMID: 10497842
Cited by
-
A Low-Cost Microfluidic-Based Detection Device for Rapid Identification and Quantification of Biomarkers-Based on a Smartphone.Biosensors (Basel). 2023 Jul 22;13(7):753. doi: 10.3390/bios13070753. Biosensors (Basel). 2023. PMID: 37504151 Free PMC article.
-
Cleavable hairpin beacon-enhanced fluorescence detection of nucleic acid isothermal amplification and smartphone-based readout.Sci Rep. 2020 Nov 2;10(1):18819. doi: 10.1038/s41598-020-75795-y. Sci Rep. 2020. PMID: 33139727 Free PMC article.
-
Honey bee sHSP are responsive to diverse proteostatic stresses and potentially promising biomarkers of honey bee stress.Sci Rep. 2021 Nov 11;11(1):22087. doi: 10.1038/s41598-021-01547-1. Sci Rep. 2021. PMID: 34764357 Free PMC article.
-
Willingness of Chinese Men Who Have Sex With Men to Use Smartphone-Based Electronic Readers for HIV Self-testing: Web-Based Cross-sectional Study.J Med Internet Res. 2021 Nov 19;23(11):e26480. doi: 10.2196/26480. J Med Internet Res. 2021. PMID: 34806988 Free PMC article.
-
Internet of medical things (IoMT)-integrated biosensors for point-of-care testing of infectious diseases.Biosens Bioelectron. 2021 May 1;179:113074. doi: 10.1016/j.bios.2021.113074. Epub 2021 Feb 6. Biosens Bioelectron. 2021. PMID: 33596516 Free PMC article. Review.
References
-
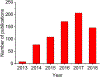
- The Statistics Portal, 2018. https://www.statista.com/statistics/274774/forecast-of-mobile-phone-user...
-
- Weinstein RS; Lopez AM; Joseph BA; Erps KA; Holcomb M; Barker GP; Krupinski EA. Am. J. Med 2014, 127, 183–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

