Efficacy and safety of low-dose Sirolimus in Lymphangioleiomyomatosis
- PMID: 30428897
- PMCID: PMC6236936
- DOI: 10.1186/s13023-018-0946-8
Efficacy and safety of low-dose Sirolimus in Lymphangioleiomyomatosis
Abstract
Background: Lymphangioleiomyomatosis is a rare disease caused by unregulated activation of mammalian target of rapamycin (mTOR) signalling pathway. Sirolimus showed efficacy in a phase 3 trial of patients with lymphangioleiomyomatosis, but the optimal dose remains unclear.
Methods: We investigated the efficacy and safety of low-dose compared with conventional-dose sirolimus. Clinical data of 39 patients with lymphangioleiomyomatosis (mean age, 34.8 years; median treatment period, 29.6 months) who received sirolimus were retrospectively reviewed. Low-dose sirolimus was defined as any dose that maintained mean blood trough levels lower than those maintained with conventional doses (5-15 ng/mL).
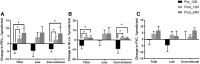
Results: Fifty-one percent of patients received low-dose therapy. The rate of decline in lung function decreased after treatment in the whole group (forced expiratory volume in 1 s [FEV1], - 0.12 ± 0.47 [before] vs. 0.24 ± 0.48% predicted/month [after], p = 0.027; diffusing capacity for carbon monoxide [DLco], - 0.33 ± 0.61 vs. 0.03 ± 0.26% predicted/month, p = 0.006) compared with before treatment. In the low-dose group, the rate of decline in FEV1 (- 0.08 ± 0.38 [before] vs. 0.19 ± 0.51% predicted/month [after], p = 0.264) and DLco (-0.13 ± 0.62 vs. 0.02 ± 0.28% predicted/month, p = 0.679) showed a numeric trend towards improvement after treatment; however, the conventional-dose group showed significant improvement in FEV1 (- 0.26 ± 0.54 [before] vs. 0.22 ± 0.38 [after] % predicted/month, p = 0.024) and DLco (- 0.55 ± 0.58 vs. 0.04 ± 0.25% predicted/month, p = 0.002) after treatment. Adverse events (AEs) occurred in 89.7% of patients and the most common AEs was hypercholesterolaemia (43.6%), followed by stomatitis (35.9%). The occurrences of AE were similar between the low- and conventional-dose groups (85.0% vs. 94.7%, p = 0.605).
Conclusions: Low-dose sirolimus may stabilise lung function decline in lymphangioleiomyomatosis patients, but its efficacy appears to be inferior to that of conventional-dose sirolimus.
Keywords: Low dose; Lymphangioleiomyomatosis; Respiratory function tests; Sirolimus; Treatment outcome.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Asan Medical Center Institutional Review Board (2016–0480).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Efficacy and safety of sirolimus in lymphangioleiomyomatosis.N Engl J Med. 2011 Apr 28;364(17):1595-606. doi: 10.1056/NEJMoa1100391. Epub 2011 Mar 16. N Engl J Med. 2011. PMID: 21410393 Free PMC article. Clinical Trial.
-
Long-term efficacy and safety of sirolimus therapy in patients with lymphangioleiomyomatosis.Orphanet J Rare Dis. 2019 Aug 20;14(1):206. doi: 10.1186/s13023-019-1178-2. Orphanet J Rare Dis. 2019. PMID: 31429781 Free PMC article.
-
Rates of change in FEV1 and DLCO as potential indicators for mTOR inhibitor therapy in premenopausal lymphangioleiomyomatosis patients.Eur Respir J. 2018 Apr 19;51(4):1702258. doi: 10.1183/13993003.02258-2017. Print 2018 Apr. Eur Respir J. 2018. PMID: 29519926 Free PMC article.
-
Current management of lymphangioleiomyomatosis.Curr Opin Pulm Med. 2011 Sep;17(5):374-8. doi: 10.1097/MCP.0b013e328349ac8c. Curr Opin Pulm Med. 2011. PMID: 21760507 Review.
-
Evolution of Diffusing Capacity of the Lungs for Carbon Monoxide in Lymphangioleiomyomatosis: Historical Perspectives and the Role of Advanced Imaging.Chest. 2025 Jun;167(6):1705-1713. doi: 10.1016/j.chest.2024.11.014. Epub 2024 Nov 21. Chest. 2025. PMID: 39580110 Review.
Cited by
-
Refractory systemic lupus erythematosus with chylous effusion successfully treated with sirolimus: a case report and literature review.Rheumatol Int. 2023 Sep;43(9):1743-1749. doi: 10.1007/s00296-023-05363-w. Epub 2023 Jun 16. Rheumatol Int. 2023. PMID: 37326666 Review.
-
Rapamycin in Cerebral Cavernous Malformations: What Doses to Test in Mice and Humans.ACS Pharmacol Transl Sci. 2022 Apr 25;5(5):266-277. doi: 10.1021/acsptsci.2c00006. eCollection 2022 May 13. ACS Pharmacol Transl Sci. 2022. PMID: 35592432 Free PMC article. Review.
-
mTOR in Lung Neoplasms.Pathol Oncol Res. 2020 Jan;26(1):35-48. doi: 10.1007/s12253-020-00796-1. Epub 2020 Feb 3. Pathol Oncol Res. 2020. PMID: 32016810 Review.
-
Lung Diseases Unique to Women.Clin Chest Med. 2021 Sep;42(3):507-516. doi: 10.1016/j.ccm.2021.04.014. Clin Chest Med. 2021. PMID: 34353455 Free PMC article. Review.
-
Rapamycin Promotes the Expansion of Myeloid Cells by Increasing G-CSF Expression in Mesenchymal Stem Cells.Front Cell Dev Biol. 2022 Mar 17;10:779159. doi: 10.3389/fcell.2022.779159. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372343 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

