Recruitment of patients with de novo Parkinson disease: successful strategies in a randomized exercise clinical trial
- PMID: 30428907
- PMCID: PMC6237042
- DOI: 10.1186/s13063-018-2958-z
Recruitment of patients with de novo Parkinson disease: successful strategies in a randomized exercise clinical trial
Abstract
Introduction: Recruitment of sufficient patients with Parkinson disease into clinical trials is a barrier to successful, timely study completion. Non-pharmacologic studies have shown to be even more challenging for recruitment, despite some studies focusing on de novo Parkinson disease populations. This paper describes successful recruitment techniques from a randomized exercise clinical trial in Parkinson disease.
Methods: Several recruitment strategies were used to enroll de novo patients with Parkinson disease into a year-long clinical trial. Strategies focused on infrastructure included fast-track clinic scheduling, weekly research meetings, an established clinical repository, real-time clinic recruitment, and outreach to the community. The nature of the study facilitated recruitment by offering a wait-listed control group, exercise at a local fitness center with a paid membership, and collection of data by shipping equipment foregoing some visits. An experienced nurse study coordinator involved in recruitment and training of the principal investigator in recruitment of minorities enhanced overall recruitment. Finally, the patient population chosen for this study, patients with de novo Parkinson disease, may be more likely to enroll in an exercise study than patients with later stage disease.
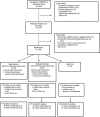
Results: Seventy-six patients with de novo Parkinson disease were successfully enrolled into the exercise clinical trial from a single site.
Conclusion: Targeted recruitment strategies were successful in this study. Additional modifications to the study protocol, such as eliminating treadmill stress tests before randomization, travel to an urban downtown location for study visits, and a relatively healthy Parkinson disease population, may also have impacted this study. These strategies could all be adopted for other studies in Parkinson disease, neurodegenerative diseases, or other chronic disorders.
Trial registration: Clinicaltrials.gov, NCT01506479 . Registered on 10 January 2012.
Keywords: Clinical trial; De novo; Parkinson disease; Recruitment; Treadmill training.
Conflict of interest statement
Ethics approval and consent to participate
The exercise RCT was approved at each participating institutions IRB – University of Colorado, University of Pittsburgh, and Rush University.
Consent for publication
All authors give consent for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2018;75(2):219–226. doi: 10.1001/jamaneurol.2017.3517. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical