A renal-cerebral-peripheral sympathetic reflex mediates insulin resistance in chronic kidney disease
- PMID: 30429087
- PMCID: PMC6286258
- DOI: 10.1016/j.ebiom.2018.10.054
A renal-cerebral-peripheral sympathetic reflex mediates insulin resistance in chronic kidney disease
Erratum in
-
Corrigendum to Cao W, Shi M, Wu L, et al. "A renal-cerebral-peripheral sympathetic reflex mediates insulin resistance in chronic kidney disease" EBioMedicine. 2018 Nov;37:281-293.EBioMedicine. 2023 Jan;87:104399. doi: 10.1016/j.ebiom.2022.104399. Epub 2022 Dec 24. EBioMedicine. 2023. PMID: 36571902 Free PMC article. No abstract available.
Abstract
Background: Insulin resistance (IR) complicates chronic kidney disease (CKD). We tested the hypothesis that CKD activates a broad reflex response from the kidneys and the white adipose tissue to impair peripheral glucose uptake and investigated the role of salt intake in this process.
Methods: 5/6-nephrectomized rats were administered normal- or high-salt for 3 weeks. Conclusions were tested in 100 non-diabetic patients with stage 3-5 CKD.
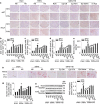
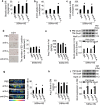
Findings: High-salt in 5/6-nephrectomized rats decreased insulin-stimulated 2-deoxyglucose uptake >25% via a sympathetic nervous system (SNS) reflex that linked the IR to reactive oxygen species (ROS) and the renin-angiotensin system (RAS) in brain and peripheral tissues. Salt-loading in CKD enhanced inflammation in adipose tissue and skeletal muscle, and enhanced the impairment of insulin signaling and Glut4 trafficking. Denervation of the kidneys or adipose tissue or deafferentation of adipose tissue improved IR >40%. In patients with non-diabetic CKD, IR was positively correlated with salt intake after controlling for cofounders (r = 0.334, P = 0.001) and was linked to activation of the RAS/SNS and to impaired glucose uptake in adipose tissue and skeletal muscle, all of which depended on salt intake.
Interpretation: CKD engages a renal/adipose-cerebral-peripheral sympathetic reflex that activates the RAS/ROS axes to promote IR via local inflammation and impaired Glut4 trafficking that are enhanced by high-salt intake. The findings point to a role for blockade of RAS or α-and-β-adrenergic receptors to reduce IR in patients with CKD. FUND: National Natural Science Foundation of China.
Keywords: Adipose tissue; Chronic kidney disease; Insulin resistance; Renin-angiotensin system; Salt; Sympathetic reflex.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures








References
-
- Shinohara K., Shoji T., Emoto M. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13(7):1894–1900. - PubMed
-
- Kaartinen K., Syrjanen J., Porsti I. Insulin resistance and the progression of IgA glomerulonephritis. Nephrol Dial Transplant. 2007;22(3):778–783. - PubMed
-
- Spoto B., Pisano A., Zoccali C. Insulin resistance in chronic kidney disease: A systematic review. Am J Physiol Renal Physiol. 2016;311(6) [F1087-F108] - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

