Modifying aroylhydrazone prochelators for hydrolytic stability and improved cytoprotection against oxidative stress
- PMID: 30429096
- PMCID: PMC6314015
- DOI: 10.1016/j.bmc.2018.11.004
Modifying aroylhydrazone prochelators for hydrolytic stability and improved cytoprotection against oxidative stress
Abstract
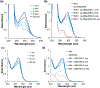
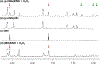
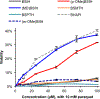
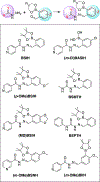
BSIH ((E)-N'-(2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzylidene)isonicotinohydrazide) is a prodrug version of the metal chelator SIH ((E)-N'-(2-hydroxybenzylidene)isonicotinohydrazide) in which a boronate group prevents metal chelation until reaction with hydrogen peroxide releases SIH, which is then available for sequestering iron(III) and inhibiting iron-catalyzed oxidative damage. While BSIH has shown promise for conditionally targeting iron sequestration in cells under oxidative stress, the yield of SIH is limited by the fact that BSIH exists in cell culture media as an equilibrium mixture with its hydrolysis products isoniazid and 2-formylphenyl boronic acid. In the current study, several BSIH analogs were evaluated for their hydrolytic stability, reaction outcomes with H2O2, and prochelator-to-chelator conversion efficiency. Notably, the para-methoxy derivative (p-OMe)BSIH ((E)-N'-(5-methoxy-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzylidene)isonicotinohydrazide) and the meta-, para-double substituted (MD)BSIH ((E)-N'-((6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d][1,3]dioxol-5-yl)methylene)isonicotinohydrazide) showed 1.3- and 1.9-fold improved hydrolytic stability compared to BSIH, respectively, leading to a 22 and 50% increase in chelator released. Moreover, both prochelators were found to protect retinal pigment epithelial cells stressed with either H2O2 or paraquat insult.
Keywords: Chelating agent; Cytoprotection; Fenton chemistry; Iron; Oxidative stress.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


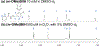



References
-
- Stockwell BR; Friedmann Angeli JP; Bayir H; Bush AI; Conrad M; Dixon SJ; Fulda S; Gascón S; Hatzios SK; Kagan VE; Noel K; Jiang X; Linkermann A; Murphy ME; Overholtzer M; Oyagi A; Pagnussat GC; Park J; Ran Q; Rosenfeld CS; Salnikow K; Tang D; Torti FM; Torti SV; Toyokuni S; Woerpel KA; Zhang DD. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017; 171: 273–285. - PMC - PubMed
-
- Zecca L; Youdim MB; Riederer P; Connor JR; Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci 2004; 5: 863–873. - PubMed
-
- Zhou T; Ma Y; Kong X; Hider RC. Design of iron chelators with therapeutic application. Dalton Trans 2012; 41: 6371–6389 - PubMed
-
- Dunaief JL. Iron induced oxidative damage as a potential factor in age-related macular degeneration: The Cogan Lecture. Invest. Ophthalmol. Vis. Sci 2006; 47: 4660–4664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

