Initial scaffold thickness affects the emergence of a geometrical and mechanical equilibrium in engineered cardiovascular tissues
- PMID: 30429259
- PMCID: PMC6283988
- DOI: 10.1098/rsif.2018.0359
Initial scaffold thickness affects the emergence of a geometrical and mechanical equilibrium in engineered cardiovascular tissues
Abstract
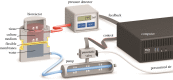
In situ cardiovascular tissue-engineering can potentially address the shortcomings of the current replacement therapies, in particular, their inability to grow and remodel. In native tissues, it is widely accepted that physiological growth and remodelling occur to maintain a homeostatic mechanical state to conserve its function, regardless of changes in the mechanical environment. A similar homeostatic state should be reached for tissue-engineered (TE) prostheses to ensure proper functioning. For in situ tissue-engineering approaches obtaining such a state greatly relies on the initial scaffold design parameters. In this study, it is investigated if the simple scaffold design parameter initial thickness, influences the emergence of a mechanical and geometrical equilibrium state in in vitro TE constructs, which resemble thin cardiovascular tissues such as heart valves and arteries. Towards this end, two sample groups with different initial thicknesses of myofibroblast-seeded polycaprolactone-bisurea constructs were cultured for three weeks under dynamic loading conditions, while tracking geometrical and mechanical changes temporally using non-destructive ultrasound imaging. A mechanical equilibrium was reached in both groups, although at different magnitudes of the investigated mechanical quantities. Interestingly, a geometrically stable state was only established in the thicker constructs, while the thinner constructs' length continuously increased. This demonstrates that reaching geometrical and mechanical stability in TE constructs is highly dependent on functional scaffold design.
Keywords: cardiovascular; growth; remodelling; tissue-engineering.
© 2018 The Author(s).
Conflict of interest statement
We have no competing interests.
Figures





Similar articles
-
Scaffold Geometry-Imposed Anisotropic Mechanical Loading Guides the Evolution of the Mechanical State of Engineered Cardiovascular Tissues in vitro.Front Bioeng Biotechnol. 2022 Feb 16;10:796452. doi: 10.3389/fbioe.2022.796452. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35252127 Free PMC article.
-
A Bioreactor to Identify the Driving Mechanical Stimuli of Tissue Growth and Remodeling.Tissue Eng Part C Methods. 2017 Jun;23(6):377-387. doi: 10.1089/ten.TEC.2017.0141. Epub 2017 May 29. Tissue Eng Part C Methods. 2017. PMID: 28478703
-
Superior Tissue Evolution in Slow-Degrading Scaffolds for Valvular Tissue Engineering.Tissue Eng Part A. 2016 Jan;22(1-2):123-32. doi: 10.1089/ten.TEA.2015.0203. Epub 2015 Dec 1. Tissue Eng Part A. 2016. PMID: 26466917
-
Current state of fabrication technologies and materials for bone tissue engineering.Acta Biomater. 2018 Oct 15;80:1-30. doi: 10.1016/j.actbio.2018.09.031. Epub 2018 Sep 22. Acta Biomater. 2018. PMID: 30248515 Review.
-
Biodegradable and biomimetic elastomeric scaffolds for tissue-engineered heart valves.Acta Biomater. 2017 Jan 15;48:2-19. doi: 10.1016/j.actbio.2016.10.032. Epub 2016 Oct 22. Acta Biomater. 2017. PMID: 27780764 Review.
Cited by
-
Surface Modification of Sponge-like Porous Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/Gelatine Blend Scaffolds for Potential Biomedical Applications.Polymers (Basel). 2022 Apr 22;14(9):1710. doi: 10.3390/polym14091710. Polymers (Basel). 2022. PMID: 35566880 Free PMC article.
-
Anisotropicity and flexibility in trilayered microfibrous substrates promote heart valve leaflet tissue engineering.Biomed Mater. 2022 Oct 7;17(6):10.1088/1748-605X/ac94ae. doi: 10.1088/1748-605X/ac94ae. Biomed Mater. 2022. PMID: 36150373 Free PMC article.
-
A mechanical modeling framework to study endothelial permeability.Biophys J. 2024 Feb 6;123(3):334-348. doi: 10.1016/j.bpj.2023.12.026. Epub 2024 Jan 1. Biophys J. 2024. PMID: 38169215 Free PMC article.
-
Scaffold Geometry-Imposed Anisotropic Mechanical Loading Guides the Evolution of the Mechanical State of Engineered Cardiovascular Tissues in vitro.Front Bioeng Biotechnol. 2022 Feb 16;10:796452. doi: 10.3389/fbioe.2022.796452. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35252127 Free PMC article.
-
Optimization of Tissue-Engineered Vascular Graft Design Using Computational Modeling.Tissue Eng Part C Methods. 2019 Oct;25(10):561-570. doi: 10.1089/ten.TEC.2019.0086. Epub 2019 Sep 3. Tissue Eng Part C Methods. 2019. PMID: 31218941 Free PMC article.
References
-
- Suma H. 1999. Arterial grafts in coronary bypass surgery. Ann. Thorac. Cardiovasc. Surg. 5, 141–145. - PubMed

