Epithelial Cell Chirality Revealed by Three-Dimensional Spontaneous Rotation
- PMID: 30429314
- PMCID: PMC6275504
- DOI: 10.1073/pnas.1805932115
Epithelial Cell Chirality Revealed by Three-Dimensional Spontaneous Rotation
Abstract
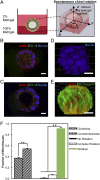
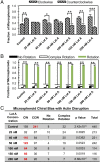
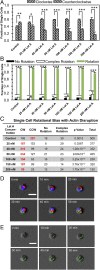
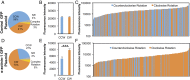
Our understanding of the left-right (LR) asymmetry of embryonic development, in particular the contribution of intrinsic handedness of the cell or cell chirality, is limited due to the confounding systematic and environmental factors during morphogenesis and a ack of physiologically relevant in vitro 3D platforms. Here we report an efficient two-layered biomaterial platform for determining the chirality of individual cells, cell aggregates, and self-organized hollow epithelial spheroids. This bioengineered niche provides a uniform defined axis allowing for cells to rotate spontaneously with a directional bias toward either clockwise or counterclockwise directions. Mechanistic studies reveal an actin-dependent, cell-intrinsic property of 3D chirality that can be mediated by actin cross-linking via α-actinin-1. Our findings suggest that the gradient of extracellular matrix is an important biophysicochemical cue influencing cell polarity and chirality. Engineered biomaterial systems can serve as an effective platform for studying developmental asymmetry and screening for environmental factors causing birth defects.
Keywords: biomaterial; cell chirality; cell polarity; left–right asymmetry; tissue morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inhibition of cell-cell adhesion impairs directional epithelial migration on micropatterned surfaces.Integr Biol (Camb). 2015 May;7(5):580-90. doi: 10.1039/c5ib00073d. Epub 2015 Apr 29. Integr Biol (Camb). 2015. PMID: 25923643
-
Cellular and Nuclear Alignment Analysis for Determining Epithelial Cell Chirality.Ann Biomed Eng. 2016 May;44(5):1475-86. doi: 10.1007/s10439-015-1431-3. Epub 2015 Aug 21. Ann Biomed Eng. 2016. PMID: 26294010 Free PMC article.
-
Chirality in planar cell shape contributes to left-right asymmetric epithelial morphogenesis.Science. 2011 Jul 15;333(6040):339-41. doi: 10.1126/science.1200940. Science. 2011. PMID: 21764746
-
Cell chirality: emergence of asymmetry from cell culture.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150413. doi: 10.1098/rstb.2015.0413. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821525 Free PMC article. Review.
-
Cell chirality: its origin and roles in left-right asymmetric development.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150403. doi: 10.1098/rstb.2015.0403. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821533 Free PMC article. Review.
Cited by
-
Cell Chirality as a Novel Measure for Cytotoxicity.Adv Biol (Weinh). 2022 Jan;6(1):e2101088. doi: 10.1002/adbi.202101088. Epub 2021 Nov 19. Adv Biol (Weinh). 2022. PMID: 34796704 Free PMC article. Review.
-
Global versus local matrix remodeling drives rotational versus invasive collective migration of epithelial cells.Dev Cell. 2025 Mar 24;60(6):871-884.e8. doi: 10.1016/j.devcel.2024.11.021. Epub 2024 Dec 19. Dev Cell. 2025. PMID: 39706188
-
Actin polymerisation and crosslinking drive left-right asymmetry in single cell and cell collectives.Nat Commun. 2023 Feb 11;14(1):776. doi: 10.1038/s41467-023-35918-1. Nat Commun. 2023. PMID: 36774346 Free PMC article.
-
Cell jamming regulates epithelial chiral morphogenesis.J Biomech. 2023 Jan;147:111435. doi: 10.1016/j.jbiomech.2023.111435. Epub 2023 Jan 5. J Biomech. 2023. PMID: 36641827 Free PMC article.
-
Cell organelle-based analysis of cell chirality.Commun Integr Biol. 2019 Apr 24;12(1):78-81. doi: 10.1080/19420889.2019.1605277. eCollection 2019. Commun Integr Biol. 2019. PMID: 31143366 Free PMC article.
References
-
- Aylsworth AS. Clinical aspects of defects in the determination of laterality. Am J Med Genet. 2001;101:345–355. - PubMed
-
- Levin M. Left-right asymmetry in embryonic development: A comprehensive review. Mech Dev. 2005;122:3–25. - PubMed
-
- Taniguchi K, et al. Chirality in planar cell shape contributes to left-right asymmetric epithelial morphogenesis. Science. 2011;333:339–341. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

