Replication-Competent NYVAC-KC Yields Improved Immunogenicity to HIV-1 Antigens in Rhesus Macaques Compared to Nonreplicating NYVAC
- PMID: 30429340
- PMCID: PMC6340019
- DOI: 10.1128/JVI.01513-18
Replication-Competent NYVAC-KC Yields Improved Immunogenicity to HIV-1 Antigens in Rhesus Macaques Compared to Nonreplicating NYVAC
Erratum in
-
Correction for Kibler et al., "Replication-Competent NYVAC-KC Yields Improved Immunogenicity to HIV-1 Antigens in Rhesus Macaques Compared to Nonreplicating NYVAC".J Virol. 2019 Oct 15;93(21):e00968-19. doi: 10.1128/JVI.00968-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31615930 Free PMC article. No abstract available.
Abstract
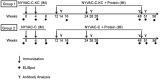
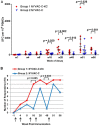
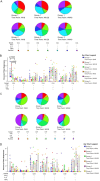
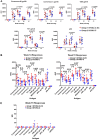
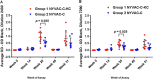
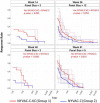
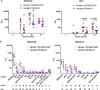
As part of the continuing effort to develop an effective HIV vaccine, we generated a poxviral vaccine vector (previously described) designed to improve on the results of the RV144 phase III clinical trial. The construct, NYVAC-KC, is a replication-competent, attenuated recombinant of the vaccinia virus strain NYVAC. NYVAC is a vector that has been used in many previous clinical studies but is replication deficient. Here, we report a side-by-side comparison of replication-restricted NYVAC and replication-competent NYVAC-KC in a nonhuman primate study, which utilized a prime-boost regimen similar to that of RV144. NYVAC-C and NYVAC-C-KC express the HIV-1 antigens gp140, and Gag/Gag-Pol-Nef-derived virus-like particles (VLPs) from clade C and were used as the prime, with recombinant virus plus envelope protein used as the boost. In nearly every T and B cell immune assay against HIV-1, including neutralization and antibody binding, NYVAC-C-KC induced a greater immune response than NYVAC-C, indicating that replication competence in a poxvirus may improve upon the modestly successful regimen used in the RV144 clinical trial.IMPORTANCE Though the RV144 phase III clinical trial showed promise that an effective vaccine against HIV-1 is possible, a successful vaccine will require improvement over the vaccine candidate (ALVAC) used in the RV144 study. With that goal in mind, we have tested in nonhuman primates an attenuated but replication-competent vector, NYVAC-KC, in direct comparison to its parental vector, NYVAC, which is replication restricted in human cells, similar to the ALVAC vector used in RV144. We have utilized a prime-boost regimen for administration of the vaccine candidate that is similar to the one used in the RV144 study. The results of this study indicate that a replication-competent poxvirus vector may improve upon the effectiveness of the RV144 clinical trial vaccine candidate.
Keywords: Gag-Pol-Nef; HIV; NYVAC; NYVAC-KC; T cell response; antibody responses; gp140; nonhuman primates; vaccines.
Copyright © 2019 American Society for Microbiology.
Figures

Similar articles
-
HIV/AIDS Vaccine Candidates Based on Replication-Competent Recombinant Poxvirus NYVAC-C-KC Expressing Trimeric gp140 and Gag-Derived Virus-Like Particles or Lacking the Viral Molecule B19 That Inhibits Type I Interferon Activate Relevant HIV-1-Specific B and T Cell Immune Functions in Nonhuman Primates.J Virol. 2017 Apr 13;91(9):e02182-16. doi: 10.1128/JVI.02182-16. Print 2017 May 1. J Virol. 2017. PMID: 28179536 Free PMC article.
-
Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost.J Virol. 2019 Jan 17;93(3):e01529-18. doi: 10.1128/JVI.01529-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429343 Free PMC article.
-
Head-to-Head Comparison of Poxvirus NYVAC and ALVAC Vectors Expressing Identical HIV-1 Clade C Immunogens in Prime-Boost Combination with Env Protein in Nonhuman Primates.J Virol. 2015 Aug;89(16):8525-39. doi: 10.1128/JVI.01265-15. Epub 2015 Jun 3. J Virol. 2015. PMID: 26041302 Free PMC article.
-
Design Concepts of Virus-Like Particle-Based HIV-1 Vaccines.Front Immunol. 2020 Sep 30;11:573157. doi: 10.3389/fimmu.2020.573157. eCollection 2020. Front Immunol. 2020. PMID: 33117367 Free PMC article. Review.
-
Is developing an HIV-1 vaccine possible?Curr Opin HIV AIDS. 2010 Sep;5(5):362-7. doi: 10.1097/COH.0b013e32833d2e90. Curr Opin HIV AIDS. 2010. PMID: 20978375 Free PMC article. Review.
Cited by
-
Intranasal Immunization with a Vaccinia Virus Vaccine Vector Expressing Pre-Fusion Stabilized SARS-CoV-2 Spike Fully Protected Mice against Lethal Challenge with the Heavily Mutated Mouse-Adapted SARS2-N501YMA30 Strain of SARS-CoV-2.Vaccines (Basel). 2022 Jul 23;10(8):1172. doi: 10.3390/vaccines10081172. Vaccines (Basel). 2022. PMID: 35893821 Free PMC article.
-
Modified Adenovirus Prime-Protein Boost Clade C HIV Vaccine Strategy Results in Reduced Viral DNA in Blood and Tissues Following Tier 2 SHIV Challenge.Front Immunol. 2021 Feb 15;11:626464. doi: 10.3389/fimmu.2020.626464. eCollection 2020. Front Immunol. 2021. PMID: 33658998 Free PMC article.
-
Heterologous Combination of VSV-GP and NYVAC Vectors Expressing HIV-1 Trimeric gp145 Env as Vaccination Strategy to Induce Balanced B and T Cell Immune Responses.Front Immunol. 2019 Dec 18;10:2941. doi: 10.3389/fimmu.2019.02941. eCollection 2019. Front Immunol. 2019. PMID: 31921191 Free PMC article.
-
A Novel MVA-Based HIV Vaccine Candidate (MVA-gp145-GPN) Co-Expressing Clade C Membrane-Bound Trimeric gp145 Env and Gag-Induced Virus-Like Particles (VLPs) Triggered Broad and Multifunctional HIV-1-Specific T Cell and Antibody Responses.Viruses. 2019 Feb 16;11(2):160. doi: 10.3390/v11020160. Viruses. 2019. PMID: 30781504 Free PMC article.
-
Repair of a previously uncharacterized second host-range gene contributes to full replication of modified vaccinia virus Ankara (MVA) in human cells.Proc Natl Acad Sci U S A. 2020 Feb 18;117(7):3759-3767. doi: 10.1073/pnas.1921098117. Epub 2020 Feb 4. Proc Natl Acad Sci U S A. 2020. PMID: 32019881 Free PMC article.
References
-
- Moss B. 1996. Poxviridae: the viruses and their replication, p 2637–2671. In Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman R, Straus SE (ed), Fields virology, 3rd ed, vol 2 Lippincott-Raven, Philadelphia, PA.
-
- Paoletti E, Tartaglia J, Taylor J. 1994. Safe and effective poxvirus vectors–NYVAC and ALVAC. Dev Biol Stand 82:65–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

