Replication-Competent NYVAC-KC Yields Improved Immunogenicity to HIV-1 Antigens in Rhesus Macaques Compared to Nonreplicating NYVAC
- PMID: 30429340
- PMCID: PMC6340019
- DOI: 10.1128/JVI.01513-18
Replication-Competent NYVAC-KC Yields Improved Immunogenicity to HIV-1 Antigens in Rhesus Macaques Compared to Nonreplicating NYVAC
Erratum in
-
Correction for Kibler et al., "Replication-Competent NYVAC-KC Yields Improved Immunogenicity to HIV-1 Antigens in Rhesus Macaques Compared to Nonreplicating NYVAC".J Virol. 2019 Oct 15;93(21):e00968-19. doi: 10.1128/JVI.00968-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31615930 Free PMC article. No abstract available.
Abstract
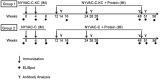
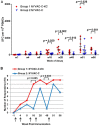
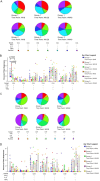
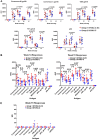
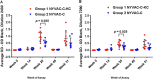
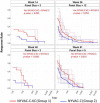
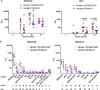
As part of the continuing effort to develop an effective HIV vaccine, we generated a poxviral vaccine vector (previously described) designed to improve on the results of the RV144 phase III clinical trial. The construct, NYVAC-KC, is a replication-competent, attenuated recombinant of the vaccinia virus strain NYVAC. NYVAC is a vector that has been used in many previous clinical studies but is replication deficient. Here, we report a side-by-side comparison of replication-restricted NYVAC and replication-competent NYVAC-KC in a nonhuman primate study, which utilized a prime-boost regimen similar to that of RV144. NYVAC-C and NYVAC-C-KC express the HIV-1 antigens gp140, and Gag/Gag-Pol-Nef-derived virus-like particles (VLPs) from clade C and were used as the prime, with recombinant virus plus envelope protein used as the boost. In nearly every T and B cell immune assay against HIV-1, including neutralization and antibody binding, NYVAC-C-KC induced a greater immune response than NYVAC-C, indicating that replication competence in a poxvirus may improve upon the modestly successful regimen used in the RV144 clinical trial.IMPORTANCE Though the RV144 phase III clinical trial showed promise that an effective vaccine against HIV-1 is possible, a successful vaccine will require improvement over the vaccine candidate (ALVAC) used in the RV144 study. With that goal in mind, we have tested in nonhuman primates an attenuated but replication-competent vector, NYVAC-KC, in direct comparison to its parental vector, NYVAC, which is replication restricted in human cells, similar to the ALVAC vector used in RV144. We have utilized a prime-boost regimen for administration of the vaccine candidate that is similar to the one used in the RV144 study. The results of this study indicate that a replication-competent poxvirus vector may improve upon the effectiveness of the RV144 clinical trial vaccine candidate.
Keywords: Gag-Pol-Nef; HIV; NYVAC; NYVAC-KC; T cell response; antibody responses; gp140; nonhuman primates; vaccines.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Moss B. 1996. Poxviridae: the viruses and their replication, p 2637–2671. In Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman R, Straus SE (ed), Fields virology, 3rd ed, vol 2 Lippincott-Raven, Philadelphia, PA.
-
- Paoletti E, Tartaglia J, Taylor J. 1994. Safe and effective poxvirus vectors–NYVAC and ALVAC. Dev Biol Stand 82:65–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

