Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost
- PMID: 30429343
- PMCID: PMC6340047
- DOI: 10.1128/JVI.01529-18
Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost
Abstract
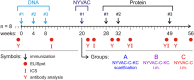
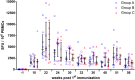
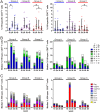
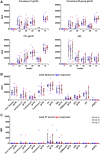
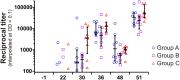
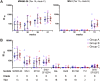
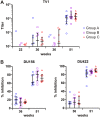
The use of heterologous immunization regimens and improved vector systems has led to increases in immunogenicity of HIV-1 vaccine candidates in nonhuman primates. In order to resolve interrelations between different delivery modalities, three different poxvirus boost regimens were compared. Three groups of rhesus macaques were each primed with the same DNA vaccine encoding Gag, Pol, Nef, and gp140. The groups were then boosted with either the vaccinia virus strain NYVAC or a variant with improved replication competence in human cells, termed NYVAC-KC. The latter was administered either by scarification or intramuscularly. Finally, macaques were boosted with adjuvanted gp120 protein to enhance humoral responses. The regimen elicited very potent CD4+ and CD8+ T cell responses in a well-balanced manner, peaking 2 weeks after the boost. T cells were broadly reactive and polyfunctional. All animals exhibited antigen-specific humoral responses already after the poxvirus boost, which further increased following protein administration. Polyclonal reactivity of IgG antibodies was highest against HIV-1 clade C Env proteins, with considerable cross-reactivity to other clades. Substantial effector functional activities (antibody-dependent cell-mediated cytotoxicity and antibody-dependent cell-mediated virus inhibition) were observed in serum obtained after the last protein boost. Notably, major differences between the groups were absent, indicating that the potent priming induced by the DNA vaccine initially framed the immune responses in such a way that the subsequent boosts with NYVAC and protein led only to an increase in the response magnitudes without skewing the quality. This study highlights the importance of selecting the best combination of vector systems in heterologous prime-boost vaccination regimens.IMPORTANCE The evaluation of HIV vaccine efficacy trials indicates that protection would most likely correlate with a polyfunctional immune response involving several effector functions from all arms of the immune system. Heterologous prime-boost regimens have been shown to elicit vigorous T cell and antibody responses in nonhuman primates that, however, qualitatively and quantitatively differ depending on the respective vector systems used. The present study evaluated a DNA prime and poxvirus and protein boost regimen and compared how two poxvirus vectors with various degrees of replication capacity and two different delivery modalities-conventional intramuscular delivery and percutaneous delivery by scarification-impact several immune effectors. It was found that despite the different poxvirus boosts, the overall immune responses in the three groups were similar, suggesting the potent DNA priming as the major determining factor of immune responses. These findings emphasize the importance of selecting optimal priming agents in heterologous prime-boost vaccination settings.
Keywords: DNA vaccine; Gag-Pol-Nef; NYVAC; NYVAC-KC; T cell responses; antibody responses; gp140; human immunodeficiency virus; nonhuman primates; vaccine.
Copyright © 2019 American Society for Microbiology.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS. 2016. AIDS by the numbers—AIDS is not over, but it can be. UNAIDS, Geneva, Switzerland.
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi:10.1056/NEJMoa0908492. - DOI - PubMed
-
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao H-X, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi:10.1056/NEJMoa1113425. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

