NF-κB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification
- PMID: 30429363
- PMCID: PMC6302947
- DOI: 10.1172/jci.insight.122958
NF-κB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification
Abstract
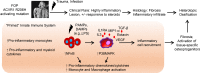
Background: Inflammation helps regulate normal growth and tissue repair. Although bone morphogenetic proteins (BMPs) and inflammation are known contributors to abnormal bone formation, how these pathways interact in ossification remains unclear.
Methods: We examined this potential link in patients with fibrodysplasia ossificans progressiva (FOP), a genetic condition of progressive heterotopic ossification caused by activating mutations in the Activin A type I receptor (ACVR1/ALK2). FOP patients show exquisite sensitivity to trauma, suggesting that BMP pathway activation may alter immune responses. We studied primary blood, monocyte, and macrophage samples from control and FOP subjects using multiplex cytokine, gene expression, and protein analyses; examined CD14+ primary monocyte and macrophage responses to TLR ligands; and assayed BMP, TGF-β activated kinase 1 (TAK1), and NF-κB pathways.
Results: FOP subjects at baseline without clinically evident heterotopic ossification showed increased serum IL-3, IL-7, IL-8, and IL-10. CD14+ primary monocytes treated with the TLR4 activator LPS showed increased CCL5, CCR7, and CXCL10; abnormal cytokine/chemokine secretion; and prolonged activation of the NF-κB pathway. FOP macrophages derived from primary monocytes also showed abnormal cytokine/chemokine secretion, increased TGF-β production, and p38MAPK activation. Surprisingly, SMAD phosphorylation was not significantly changed in the FOP monocytes/macrophages.
Conclusions: Abnormal ACVR1 activity causes a proinflammatory state via increased NF-κB and p38MAPK activity. Similar changes may contribute to other types of heterotopic ossification, such as in scleroderma and dermatomyositis; after trauma; or with recombinant BMP-induced bone fusion. Our findings suggest that chronic antiinflammatory treatment may be useful for heterotopic ossification.
Keywords: Bone Biology; Bone disease; Cellular immune response; Genetic diseases; Inflammation.
Conflict of interest statement
Figures




References
-
- Frangogiannis NG. The Inflammatory Response in Tissue Repair. In: Cavaillon JM, Singer M, eds. Inflammation: From Molecular and Cellular Mechanisms to the Clinic. Hoboken, NJ: Wiley; 2017:1517–1537.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

