Metal-free ribonucleotide reduction powered by a DOPA radical in Mycoplasma pathogens
- PMID: 30429545
- PMCID: PMC6317698
- DOI: 10.1038/s41586-018-0653-6
Metal-free ribonucleotide reduction powered by a DOPA radical in Mycoplasma pathogens
Abstract
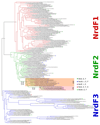
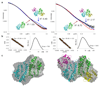
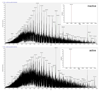
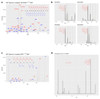
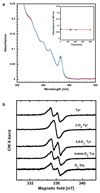
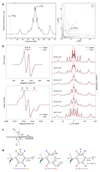
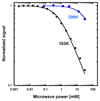
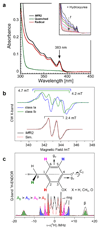
Ribonucleotide reductase (RNR) catalyses the only known de novo pathway for the production of all four deoxyribonucleotides that are required for DNA synthesis1,2. It is essential for all organisms that use DNA as their genetic material and is a current drug target3,4. Since the discovery that iron is required for function in the aerobic, class I RNR found in all eukaryotes and many bacteria, a dinuclear metal site has been viewed as necessary to generate and stabilize the catalytic radical that is essential for RNR activity5-7. Here we describe a group of RNR proteins in Mollicutes-including Mycoplasma pathogens-that possess a metal-independent stable radical residing on a modified tyrosyl residue. Structural, biochemical and spectroscopic characterization reveal a stable 3,4-dihydroxyphenylalanine (DOPA) radical species that directly supports ribonucleotide reduction in vitro and in vivo. This observation overturns the presumed requirement for a dinuclear metal site in aerobic ribonucleotide reductase. The metal-independent radical requires new mechanisms for radical generation and stabilization, processes that are targeted by RNR inhibitors. It is possible that this RNR variant provides an advantage under metal starvation induced by the immune system. Organisms that encode this type of RNR-some of which are developing resistance to antibiotics-are involved in diseases of the respiratory, urinary and genital tracts. Further characterization of this RNR family and its mechanism of cofactor generation will provide insight into new enzymatic chemistry and be of value in devising strategies to combat the pathogens that utilize it. We propose that this RNR subclass is denoted class Ie.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. - PubMed
-
- Aye Y, Li M, Long MJC, Weiss RS. Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene. 2015;34:2011–2021. - PubMed
-
- Brown NC, Eliasson R, Reichard P, Thelander L. Nonheme iron as a cofactor in ribonucleotide reductase from E. coli. Biochem Biophys Res Commun. 1968;30:522–527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

