In vitro conversion of adult murine endothelial cells to hematopoietic stem cells
- PMID: 30429596
- PMCID: PMC9923715
- DOI: 10.1038/s41596-018-0060-3
In vitro conversion of adult murine endothelial cells to hematopoietic stem cells
Abstract
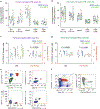
The ability to generate hematopoietic stem cells (HSCs) in vitro would have an immeasurable impact on many areas of clinical practice, including trauma, cancer, and congenital disease. In this protocol, we describe a stepwise approach that converts adult murine endothelial cells (ECs) to HSCs, termed 'reprogrammed ECs into hematopoietic stem and progenitor cells' (rEC-HSPCs). The conversion, which is achieved without cells transitioning through a pluripotent state, comprises three phases: induction, specification, and expansion. Adult ECs are first isolated from Runx1-IRES-GFP; Rosa26-rtTa mice and maintained in culture under EC growth factor stimulation and Tgfβ inhibition. In the first (induction) phase of conversion (days 0-8), four transcription factors (TFs)-FosB, Gfi1, Runx1, and Spi1 (FGRS)-are expressed transiently, which results in endogenous Runx1 expression. During the second (specification) phase (days 8-20), endogenous Runx1+ FGRS-transduced ECs commit to a hematopoietic fate and no longer require exogenous FGRS expression. Finally, the vascular niche drives robust proliferation of rEC-HSPCs during the expansion phase (days 20-28). The resulting converted cells possess a transcriptomic signature and long-term self-renewal capacity indistinguishable from those of adult HSCs. In this protocol, we also describe functional in vitro and in vivo assays that can be used to demonstrate that rEC-HSPCs are competent for clonal engraftment and possess multi-lineage reconstitution potential, including antigen-dependent adaptive immune function. This approach thus provides a tractable strategy for interrogating the generation of engraftable hematopoietic cells, advancing the mechanistic understanding of hematopoietic development and HSC self-renewal.
Conflict of interest statement
Competing Interests Statement
S.R. is the founder and a non-paid consultant to Angiocrine Bioscience, New York, New York, USA.
Figures


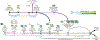
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

