Omalizumab improves forced expiratory volume in 1 second in patients with severe asthma
- PMID: 30429708
- PMCID: PMC6232554
- DOI: 10.5114/ada.2018.77241
Omalizumab improves forced expiratory volume in 1 second in patients with severe asthma
Abstract
Introduction: Asthma is a multiphenotypic disease, and therapeutic managenment in patients with severe asthma is particulary difficult, with conventional treatment of severe asthma showing poor efficacy.
Aim: To analyse forced expiratory volume in 1 s (FEV1) following the adminstration of omalizumab.
Material and methods: Six patinents (mean age: 50 ±12.6) with severe, uncontrolled asthma according to the GINA guidelines were enrolled in the study.
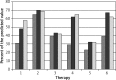
Results: Treatment with omalizumab increased in all subjects FEV1 by 17.28 ±13.4% after months and 18.57 ±13.4% after 12 months of treatment.
Conclusions: These results provides further evidence that therapy with omalizumab improves spiromtric parameters in severe asthma.
Keywords: asthma; forced expiratory volume in 1 s; omalizumab; spirometry.
Figures
Similar articles
-
Omalizumab Treatment for Atopic Severe Persistant Asthma: A Single-Center, Long-Term, Real-Life Experience with 38 Patients.Turk Thorac J. 2018 Sep 13;19(4):187-192. doi: 10.5152/TurkThoracJ.2018.17109. Print 2018 Oct. Turk Thorac J. 2018. PMID: 30322442 Free PMC article.
-
Assessment of long-term omalizumab treatment in patients with severe allergic asthma long-term omalizumab treatment in severe asthma.J Asthma. 2013 Aug;50(6):687-94. doi: 10.3109/02770903.2013.792348. Epub 2013 May 9. J Asthma. 2013. PMID: 23557459
-
Effects of omalizumab in severe asthmatics across ages: A real life Italian experience.Respir Med. 2016 Oct;119:141-149. doi: 10.1016/j.rmed.2016.09.005. Epub 2016 Sep 4. Respir Med. 2016. PMID: 27692136
-
Benefit of switching to mepolizumab from omalizumab in severe eosinophilic asthma based on patient characteristics.Respir Res. 2021 May 10;22(1):144. doi: 10.1186/s12931-021-01733-9. Respir Res. 2021. PMID: 33971856 Free PMC article. Clinical Trial.
-
Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE.Allergy. 2005 Mar;60(3):309-16. doi: 10.1111/j.1398-9995.2004.00772.x. Allergy. 2005. PMID: 15679715 Clinical Trial.
Cited by
-
Four-week IgE/baseline IgE ratio combined with tryptase predicts clinical outcome in omalizumab-treated children with moderate-to-severe asthma.Open Med (Wars). 2025 May 21;20(1):20251176. doi: 10.1515/med-2025-1176. eCollection 2025. Open Med (Wars). 2025. PMID: 40417314 Free PMC article.
References
-
- Global Initiative for Asthma (GINA) National Heart, Lung and Blood Institute (NHLBI) Global strategy for asthma management and prevention Bethesda (MD): Global Initiative for Asthma (GINA), National Heart, Lung and Blood Institute (NHLBI) 2006. Available from: www.ginasthma.com.
-
- Busse W, Corren J, Lanier BQ, et al. Omalizumab, antiIgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90. - PubMed
LinkOut - more resources
Full Text Sources