Omalizumab improves forced expiratory volume in 1 second in patients with severe asthma
- PMID: 30429708
- PMCID: PMC6232554
- DOI: 10.5114/ada.2018.77241
Omalizumab improves forced expiratory volume in 1 second in patients with severe asthma
Abstract
Introduction: Asthma is a multiphenotypic disease, and therapeutic managenment in patients with severe asthma is particulary difficult, with conventional treatment of severe asthma showing poor efficacy.
Aim: To analyse forced expiratory volume in 1 s (FEV1) following the adminstration of omalizumab.
Material and methods: Six patinents (mean age: 50 ±12.6) with severe, uncontrolled asthma according to the GINA guidelines were enrolled in the study.
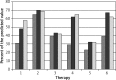
Results: Treatment with omalizumab increased in all subjects FEV1 by 17.28 ±13.4% after months and 18.57 ±13.4% after 12 months of treatment.
Conclusions: These results provides further evidence that therapy with omalizumab improves spiromtric parameters in severe asthma.
Keywords: asthma; forced expiratory volume in 1 s; omalizumab; spirometry.
Figures
References
-
- Global Initiative for Asthma (GINA) National Heart, Lung and Blood Institute (NHLBI) Global strategy for asthma management and prevention Bethesda (MD): Global Initiative for Asthma (GINA), National Heart, Lung and Blood Institute (NHLBI) 2006. Available from: www.ginasthma.com.
-
- Busse W, Corren J, Lanier BQ, et al. Omalizumab, antiIgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90. - PubMed
LinkOut - more resources
Full Text Sources