Qingchang Suppository Ameliorates Colonic Vascular Permeability in Dextran-Sulfate-Sodium-Induced Colitis
- PMID: 30429788
- PMCID: PMC6220057
- DOI: 10.3389/fphar.2018.01235
Qingchang Suppository Ameliorates Colonic Vascular Permeability in Dextran-Sulfate-Sodium-Induced Colitis
Abstract
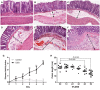
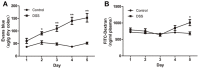
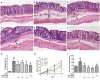
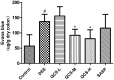
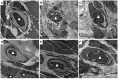
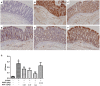
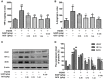
Ulcerative colitis (UC), with a long course and repeated attack, severely affects patient's life quality and increases economic burden all over the world. However, the concrete causes and mechanisms of UC are still unclear, but it is generally considered that many factors participate in this process. Qingchang Suppository (QCS) has been used in treating rectitis and colitis for about 30 years in Shanghai, China. Its satisfactory clinical effects have been proved. The aim of this study is to investigate the effect and mechanisms of QCS on colonic vascular endothelial barrier in dextran sulfate sodium (DSS)-induced colitis. The results indicated that increased vascular permeability (VP) appeared earlier than increased intestinal epithelial permeability (EP) in the process of DSS-induced colitis. QCS attenuated colonic tissue edema, vascular congestion and inflammatory cell infiltration. QCS inhibited the elevation of MPO, TNF-α, and IL-6 levels in colon tissues and alleviated the microvascular damage induced by DSS. QCS also improved colonic hypoxia and decreased the expression of VEGF, HIF-1α, and iNOS. These results revealed that QCS can reduce colonic VP and can improve vascular endothelial barrier function maybe by regulating the VEGF/HIF-1α signaling pathway.
Keywords: epithelial permeability; qingchang suppository; ulcerative colitis; vascular endothelial barrier; vascular permeability.
Figures

Similar articles
-
[Effect of Qingchang Suppository on intestinal permeability in rats with ulcerative colitis].Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010 Oct;30(10):1087-90. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010. PMID: 21066896 Chinese.
-
Anti-inflammation effect of Qingchang suppository in ulcerative colitis through JAK2/STAT3 signaling pathway in vitro and in vivo.J Ethnopharmacol. 2021 Feb 10;266:113442. doi: 10.1016/j.jep.2020.113442. Epub 2020 Oct 4. J Ethnopharmacol. 2021. PMID: 33027643
-
Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-κB activation.World J Gastroenterol. 2017 Feb 21;23(7):1180-1188. doi: 10.3748/wjg.v23.i7.1180. World J Gastroenterol. 2017. PMID: 28275298 Free PMC article.
-
Mechanism of Qingchang Suppository on repairing the intestinal mucosal barrier in ulcerative colitis.Front Pharmacol. 2023 Aug 22;14:1221849. doi: 10.3389/fphar.2023.1221849. eCollection 2023. Front Pharmacol. 2023. PMID: 37675045 Free PMC article. Review.
-
Novel pharmacologic approaches to the prevention and treatment of ulcerative colitis.Curr Pharm Des. 2013;19(1):17-28. doi: 10.2174/13816128130105. Curr Pharm Des. 2013. PMID: 22950505 Review.
Cited by
-
Notoginsenoside R2 induces colonic microvascular injuries via regulating the Rap1GAP/PI3K/Akt signaling pathway.Ann Transl Med. 2021 Dec;9(23):1743. doi: 10.21037/atm-21-5898. Ann Transl Med. 2021. PMID: 35071437 Free PMC article.
-
Pulchinenoside B4 ameliorates oral ulcers in rats by modulating gut microbiota and metabolites.Appl Microbiol Biotechnol. 2024 Apr 9;108(1):292. doi: 10.1007/s00253-024-13099-1. Appl Microbiol Biotechnol. 2024. PMID: 38592514 Free PMC article.
-
Dandelion polysaccharide treatment protects against dextran sodium sulfate-induced colitis by suppressing NF-κB/NLRP3 inflammasome-mediated inflammation and activating Nrf2 in mouse colon.Food Sci Nutr. 2023 Sep 19;11(11):7271-7282. doi: 10.1002/fsn3.3653. eCollection 2023 Nov. Food Sci Nutr. 2023. PMID: 37970386 Free PMC article.
-
Huangkui Lianchang Decoction Ameliorates DSS-Induced Ulcerative Colitis in Mice by Inhibiting the NF-kappaB Signaling Pathway.Evid Based Complement Alternat Med. 2019 Apr 10;2019:1040847. doi: 10.1155/2019/1040847. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31093294 Free PMC article.
-
Mitigation of DSS-Induced Colitis Potentially via Th1/Th2 Cytokine and Immunological Function Balance Induced by Phenolic-Enriched Buckwheat (Fagopyrum esculentum Moench) Bee Pollen Extract.Foods. 2022 Apr 29;11(9):1293. doi: 10.3390/foods11091293. Foods. 2022. PMID: 35564016 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

