PGE2 Augments Inflammasome Activation and M1 Polarization in Macrophages Infected With Salmonella Typhimurium and Yersinia enterocolitica
- PMID: 30429830
- PMCID: PMC6220063
- DOI: 10.3389/fmicb.2018.02447
PGE2 Augments Inflammasome Activation and M1 Polarization in Macrophages Infected With Salmonella Typhimurium and Yersinia enterocolitica
Abstract
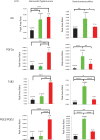
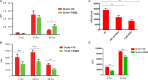
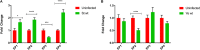
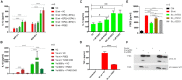
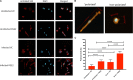
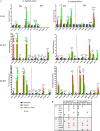
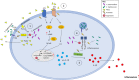
Eicosanoids are cellular metabolites, which shape the immune response, including inflammatory processes in macrophages. The effects of these lipid mediators on inflammation and bacterial pathogenesis are not clearly understood. Certain eicosanoids are suspected to act as molecular sensors for the recruitment of neutrophils, while others regulate bacterial uptake. In this study, gene expression analyses indicated that genes involved in eicosanoid biosynthesis including COX-1, COX-2, DAGL, and PLA-2 are differentially regulated in THP-1 human macrophages infected with Salmonella enterica Typhimurium or Yersinia enterocolitica. By using targeted metabolomics approach, we found that the eicosanoid precursor, arachidonic acid (AA) as well as its derivatives, including prostaglandins (PGs) PGF2α or PGE2/PGD2, and thromboxane TxB2, are rapidly secreted from macrophages infected with these Gram-negative pathogenic bacteria. The magnitude of eicosanoid biosynthesis in infected host cells depends on the presence of virulence factors of Y. enterocolitica and S. Typhimurium strains, albeit in an opposite way in Y. enterocolitica compared to S. Typhimurium infection. Trials with combinations of EP2/EP4 PGE2 receptor agonists and antagonists suggest that PGE2 signaling in these infection models works primarily through the EP4 receptor. Downstream of EP4 activation, PGE2 enhances inflammasome activation and represses M2 macrophage polarization while inducing key M1-type markers. PGE2 also led to a decreased numbers of Y. enterocolitica within macrophages. To summarize, PGE2 is a potent autocrine/paracrine activator of inflammation during infection in Gram-negative bacteria, and it affects macrophage polarization, likely controlling bacterial clearance by macrophages.
Keywords: Salmonella enterica Typhimurium; Yersinia enterocolitica; eicosanoids; inflammasome; macrophage polarization.
Figures


Similar articles
-
Yersinia pseudotuberculosis YopJ Limits Macrophage Response by Downregulating COX-2-Mediated Biosynthesis of PGE2 in a MAPK/ERK-Dependent Manner.Microbiol Spectr. 2021 Sep 3;9(1):e0049621. doi: 10.1128/Spectrum.00496-21. Epub 2021 Jul 28. Microbiol Spectr. 2021. PMID: 34319170 Free PMC article.
-
Cardiolipin Biosynthesis Genes Are Not Required for Salmonella enterica Serovar Typhimurium Pathogenesis in C57BL/6J Mice.Microbiol Spectr. 2022 Jun 29;10(3):e0261721. doi: 10.1128/spectrum.02617-21. Epub 2022 May 31. Microbiol Spectr. 2022. PMID: 35638781 Free PMC article.
-
General and specific host responses to bacterial infection in Peyer's patches: a role for stromelysin-1 (matrix metalloproteinase-3) during Salmonella enterica infection.Mol Microbiol. 2007 Apr;64(1):94-110. doi: 10.1111/j.1365-2958.2007.05635.x. Mol Microbiol. 2007. PMID: 17376075
-
Recent findings about the Yersinia enterocolitica phage shock protein response.J Microbiol. 2012 Feb;50(1):1-7. doi: 10.1007/s12275-012-1578-7. Epub 2012 Feb 27. J Microbiol. 2012. PMID: 22367931 Free PMC article. Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
The Dysregulation of Essential Fatty Acid (EFA) Metabolism May Be a Factor in the Pathogenesis of Sepsis.Medicina (Kaunas). 2024 Jun 3;60(6):934. doi: 10.3390/medicina60060934. Medicina (Kaunas). 2024. PMID: 38929553 Free PMC article. Review.
-
Exosomes released by breast cancer cells under mild hyperthermic stress possess immunogenic potential and modulate polarization in vitro in macrophages.Int J Hyperthermia. 2020;37(1):696-710. doi: 10.1080/02656736.2020.1778800. Int J Hyperthermia. 2020. PMID: 32568583 Free PMC article.
-
Dynamics of macrophage polarization support Salmonella persistence in a whole living organism.Elife. 2024 Jan 15;13:e89828. doi: 10.7554/eLife.89828. Elife. 2024. PMID: 38224094 Free PMC article.
-
Assessing immune hepatotoxicity of troglitazone with a versatile liver-immune-microphysiological-system.Front Pharmacol. 2024 May 30;15:1335836. doi: 10.3389/fphar.2024.1335836. eCollection 2024. Front Pharmacol. 2024. PMID: 38873410 Free PMC article.
-
Phagocytes produce prostaglandin E2 in response to cytosolic Listeria monocytogenes.PLoS Pathog. 2021 Sep 23;17(9):e1009493. doi: 10.1371/journal.ppat.1009493. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34555127 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

