Protein Tyrosine Phosphatases: Regulators of CD4 T Cells in Inflammatory Bowel Disease
- PMID: 30429852
- PMCID: PMC6220082
- DOI: 10.3389/fimmu.2018.02504
Protein Tyrosine Phosphatases: Regulators of CD4 T Cells in Inflammatory Bowel Disease
Abstract
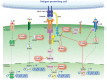
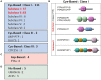
Protein tyrosine phosphatases (PTPs) play a critical role in co-ordinating the signaling networks that maintain lymphocyte homeostasis and direct lymphocyte activation. By dephosphorylating tyrosine residues, PTPs have been shown to modulate enzyme activity and both mediate and disrupt protein-protein interactions. Through these molecular mechanisms, PTPs ultimately impact lymphocyte responses to environmental cues such as inflammatory cytokines and chemokines, as well as antigenic stimulation. Mouse models of acute and chronic intestinal inflammation have been shown to be exacerbated in the absence of PTPs such as PTPN2 and PTPN22. This increase in disease severity is due in part to hyper-activation of lymphocytes in the absence of PTP activity. In accordance, human PTPs have been linked to intestinal inflammation. Genome wide association studies (GWAS) identified several PTPs within risk loci for inflammatory bowel disease (IBD). Therapeutically targeting PTP substrates and their associated signaling pathways, such as those implicated in CD4+ T cell responses, has demonstrated clinical efficacy. The current review focuses on the role of PTPs in controlling CD4+ T cell activity in the intestinal mucosa and how disruption of PTP activity in CD4+ T cells can contribute to intestinal inflammation.
Keywords: CD4 T cells; JAK-STAT; cytokine; inflammatory bowel disease; protein tyrosine phosphatase.
Figures

Similar articles
-
Role of protein tyrosine phosphatases in regulating the immune system: implications for chronic intestinal inflammation.Inflamm Bowel Dis. 2015 Mar;21(3):645-55. doi: 10.1097/MIB.0000000000000297. Inflamm Bowel Dis. 2015. PMID: 25581833 Free PMC article. Review.
-
Critical role of ROCK2 activity in facilitating mucosal CD4+ T cell activation in inflammatory bowel disease.J Autoimmun. 2018 May;89:125-138. doi: 10.1016/j.jaut.2017.12.009. Epub 2017 Dec 19. J Autoimmun. 2018. PMID: 29269245
-
Regulation of CD4+ T Cell Signaling and Immunological Synapse by Protein Tyrosine Phosphatases: Molecular Mechanisms in Autoimmunity.Front Immunol. 2019 Jun 26;10:1447. doi: 10.3389/fimmu.2019.01447. eCollection 2019. Front Immunol. 2019. PMID: 31297117 Free PMC article. Review.
-
Role of Protein Tyrosine Phosphatases in Inflammatory Bowel Disease, Celiac Disease and Diabetes: Focus on the Intestinal Mucosa.Cells. 2024 Nov 29;13(23):1981. doi: 10.3390/cells13231981. Cells. 2024. PMID: 39682729 Free PMC article. Review.
-
Intestinal epithelial cells from inflammatory bowel disease patients preferentially stimulate CD4+ T cells to proliferate and secrete interferon-gamma.Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1630-40. doi: 10.1152/ajpgi.00294.2006. Epub 2007 Mar 8. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17347451
Cited by
-
T Cell Protein Tyrosine Phosphatase in Osteoimmunology.Front Immunol. 2021 Feb 22;12:620333. doi: 10.3389/fimmu.2021.620333. eCollection 2021. Front Immunol. 2021. PMID: 33692794 Free PMC article. Review.
-
SimiC enables the inference of complex gene regulatory dynamics across cell phenotypes.Commun Biol. 2022 Apr 12;5(1):351. doi: 10.1038/s42003-022-03319-7. Commun Biol. 2022. PMID: 35414121 Free PMC article.
-
Protein tyrosine phosphatases as emerging targets for cancer immunotherapy.Br J Pharmacol. 2023 Dec 20:10.1111/bph.16304. doi: 10.1111/bph.16304. Online ahead of print. Br J Pharmacol. 2023. PMID: 38116815 Free PMC article. Review.
-
Transcriptomic profiling of severe and critical COVID-19 patients reveals alterations in expression, splicing and polyadenylation.Sci Rep. 2025 Apr 18;15(1):13469. doi: 10.1038/s41598-025-95905-y. Sci Rep. 2025. PMID: 40251257 Free PMC article.
-
Splicing QTL mapping in stimulated macrophages associates low-usage splice junctions with immune-mediated disease risk.Nat Commun. 2025 Aug 27;16(1):7205. doi: 10.1038/s41467-025-61669-2. Nat Commun. 2025. PMID: 40866368 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

