Early Induction of Human Regulatory Dermal Antigen Presenting Cells by Skin-Penetrating Schistosoma Mansoni Cercariae
- PMID: 30429854
- PMCID: PMC6220649
- DOI: 10.3389/fimmu.2018.02510
Early Induction of Human Regulatory Dermal Antigen Presenting Cells by Skin-Penetrating Schistosoma Mansoni Cercariae
Abstract
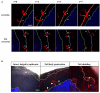
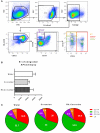
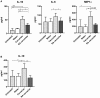
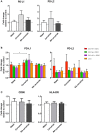
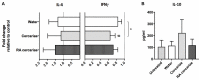
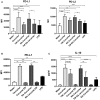
Following initial invasion of Schistosoma mansoni cercariae, schistosomula reside in the skin for several days during which they can interact with the dermal immune system. While murine experiments have indicated that exposure to radiation-attenuated (RA) cercariae can generate protective immunity which is initiated in the skin stage, contrasting non-attenuated cercariae, such data is missing for the human model. Since murine skin does not form a reliable marker for immune responses in human skin, we used human skin explants to study the interaction with non-attenuated and RA cercariae with dermal innate antigen presenting cells (APCs) and the subsequent immunological responses. We exposed human skin explants to cercariae and visualized their invasion in real time (initial 30 min) using novel imaging technologies. Subsequently, we studied dermal immune responses and found an enhanced production of regulatory cytokine interleukin (IL)-10, pro-inflammatory cytokine IL-6 and macrophage inflammatory protein (MIP)-1α within 3 days of exposure. Analysis of dermal dendritic cells (DDCs) for their phenotype revealed an increased expression of immune modulators programmed death ligand (PD-L) 1 and 2, and increased IL-10 production. Ex vivo primed DDCs suppress Th1 polarization of naïve T-cells and increase T-cell IL-10 production, indicating their regulatory potential. These immune responses were absent or decreased after exposure to RA parasites. Using transwells, we show that direct contact between APCs and cercariae is required to induce their regulatory phenotype. To the best of our knowledge this is the first study that attempts to provide insight in the human dermal S. mansoni cercariae invasion and subsequent immune responses comparing non-attenuated with RA parasites. We reveal that cercariae induce a predominantly regulatory immune response whereas RA cercariae fail to achieve this. This initial understanding of the dermal immune suppressive capacity of S. mansoni cercariae in humans provides a first step toward the development of an effective schistosome vaccine.
Keywords: PD-L1; Schistosoma mansoni; bilharzia; cercaria; human; macrophage; schistosome; skin.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

