High-Dimensional Profiling Reveals Heterogeneity of the Th17 Subset and Its Association With Systemic Immunomodulatory Treatment in Non-infectious Uveitis
- PMID: 30429855
- PMCID: PMC6220365
- DOI: 10.3389/fimmu.2018.02519
High-Dimensional Profiling Reveals Heterogeneity of the Th17 Subset and Its Association With Systemic Immunomodulatory Treatment in Non-infectious Uveitis
Abstract
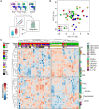
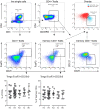
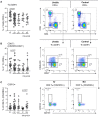
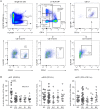
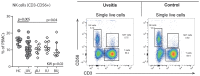
Background: Non-infectious uveitis (NIU) is a severe intra ocular inflammation, which frequently requires prompt systemic immunosuppressive therapy (IMT) to halt the development of vision-threatening complications. IMT is considered when NIU cannot be treated with corticosteroids alone, which is unpredictable in advance. Previous studies have linked blood cell subsets to glucocorticoid sensitivity, which suggests that the composition of blood leukocytes may early identify patients that will require IMT. Objective: To map the blood leukocyte composition of NIU and identify cell subsets that stratify patients that required IMT during follow-up. Methods: We performed controlled flow cytometry experiments measuring a total of 37 protein markers in the blood of 30 IMT free patients with active non-infectious anterior, intermediate, and posterior uveitis, and compared these to 15 age and sex matched healthy controls. Results from manual gating were validated by automatic unsupervised gating using FlowSOM. Results: Patients with uveitis displayed lower relative frequencies of Natural Killer cells and higher relative frequencies of memory T cells, in particular the CCR6+ lineages. These results were confirmed by automatic gating by unsupervised clustering using FlowSOM. We observed considerable heterogeneity in memory T cell subsets and abundance of CXCR3-CCR6+ (Th17) cells between the uveitis subtypes. Importantly, regardless of the uveitis subtype, patients that eventually required IMT in the course of the study follow-up exhibited increased CCR6+ T cell abundance before commencing therapy. Conclusion: High-dimensional immunoprofiling in NIU patients shows that clinically distinct forms of human NIU exhibit shared as well as unique immune cell perturbations in the peripheral blood and link CCR6+ T cell abundance to systemic immunomodulatory treatment.
Keywords: CCR6; Th17; flow cytometry; immunosuppressive therapy; non-infectious uveitis.
Figures



Similar articles
-
Clinical Remission of Sight-Threatening Non-Infectious Uveitis Is Characterized by an Upregulation of Peripheral T-Regulatory Cell Polarized Towards T-bet and TIGIT.Front Immunol. 2018 May 3;9:907. doi: 10.3389/fimmu.2018.00907. eCollection 2018. Front Immunol. 2018. PMID: 29774027 Free PMC article.
-
Coexpression of CCR6 and CD146 (MCAM) is a marker of effector memory T-helper 17 cells.J Dermatol. 2012 Oct;39(10):838-42. doi: 10.1111/j.1346-8138.2012.01544.x. Epub 2012 Apr 5. J Dermatol. 2012. PMID: 22486269
-
Clinical characteristics of non-infectious uveitis treated with and without systemic immunomodulatory therapy.Can J Ophthalmol. 2024 Oct;59(5):358-366. doi: 10.1016/j.jcjo.2023.10.002. Epub 2023 Nov 13. Can J Ophthalmol. 2024. PMID: 37972648
-
The Role of Corticosteroids and Immunomodulatory Therapy in the Management of Infectious Uveitis.Ocul Immunol Inflamm. 2024 Oct;32(8):1832-1843. doi: 10.1080/09273948.2023.2296032. Epub 2024 Feb 8. Ocul Immunol Inflamm. 2024. PMID: 38330155 Review.
-
COVID-19 and the Use of Immunomodulatory Agents in Ophthalmology.Turk J Ophthalmol. 2021 Aug 27;51(4):231-242. doi: 10.4274/tjo.galenos.2021.68252. Turk J Ophthalmol. 2021. PMID: 34461710 Free PMC article. Review.
Cited by
-
Mucosal-associated invariant T cells have therapeutic potential against ocular autoimmunity.Mucosal Immunol. 2022 Feb;15(2):351-361. doi: 10.1038/s41385-021-00469-5. Epub 2021 Nov 13. Mucosal Immunol. 2022. PMID: 34775490
-
Computational Analysis of Multiparametric Flow Cytometric Data to Dissect B Cell Subsets in Vaccine Studies.Cytometry A. 2020 Mar;97(3):259-267. doi: 10.1002/cyto.a.23922. Epub 2019 Nov 11. Cytometry A. 2020. PMID: 31710181 Free PMC article.
-
Single-cell profiling identifies a CD8bright CD244bright Natural Killer cell subset that reflects disease activity in HLA-A29-positive birdshot chorioretinopathy.Nat Commun. 2024 Jul 31;15(1):6443. doi: 10.1038/s41467-024-50472-0. Nat Commun. 2024. PMID: 39085199 Free PMC article.
-
Parameter optimization for stable clustering using FlowSOM: a case study from CyTOF.Front Immunol. 2024 Oct 9;15:1414400. doi: 10.3389/fimmu.2024.1414400. eCollection 2024. Front Immunol. 2024. PMID: 39445014 Free PMC article.
-
CRB1-Associated Retinal Dystrophy Patients Have Expanded Lewis Glycoantigen-Positive T Cells.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):6. doi: 10.1167/iovs.64.13.6. Invest Ophthalmol Vis Sci. 2023. PMID: 37792335 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

