Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing
- PMID: 30429884
- PMCID: PMC6217057
- DOI: 10.7150/thno.26837
Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing
Abstract
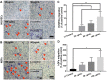
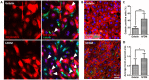
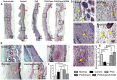
Skin injuries are frequently encountered in daily life, but deep wounds often poorly self-heal and do not recover completely. In this study, we propose a novel skin patch that combines antibiotic, cell-derived extracellular matrix (ECM) and biocompatible polyvinyl alcohol (PVA) hydrogel. Methods: Decellularized human lung fibroblast-derived matrix (hFDM) was prepared on tissue culture plate (TCP) and PVA solution was then poured onto it. After a freeze-thaw process, PVA was peeled off from TCP along with hFDM tightly anchored to PVA. Subsequently, ciprofloxacin (Cipro)-incorporated PVA/hFDM (PVA/Cipro/hFDM) was fabricated via diffusion-based drug loading. Results: In vitro analyses of PVA/Cipro/hFDM show little cytotoxicity of ciprofloxacin, stability of hFDM, rich fibronectin in hFDM, and good cell attachment, respectively. In addition, hFDM proved to be beneficial in promoting cell migration of dermal fibroblasts and human umbilical vein endothelial cells (HUVECs) using transwell inserts. The antibacterial drug Cipro was very effective in suppressing colony growth of gram-negative and -positive bacteria as identified via an inhibition zone assay. For animal study, infected wound models in BALB/c mice were prepared and four test groups (control, PVA, PVA/Cipro, PVA/Cipro/hFDM) were administered separately and their effect on wound healing was examined for up to 21 days. The results support that Cipro successfully reduced bacterial infection and thus encouraged faster wound closure. Further analysis using histology and immunofluorescence revealed that the most advanced skin regeneration was achieved with PVA/Cipro/hFDM, as assessed via re-epithelialization, collagen texture and distribution in the epidermis, and skin adnexa (i.e., glands and hair follicles) regeneration in the dermis. Conclusion: This work demonstrates that our skin patch successfully consolidates the regenerative potential of ECM and the antibacterial activity of Cipro for advanced wound healing.
Keywords: ciprofloxacin; human fibroblast-derived matrix; polyvinyl alcohol hydrogel; skin patch; wound healing.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Deng CM, He LZ, Zhao M. et al. Biological properties of the chitosan-gelatin sponge wound dressing. Carbohydr Polym. 2007;69:583–89.
-
- Fan Z, Liu B, Wang J. et al. A novel wound dressing based on Ag/graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing. Adv Funct Mater. 2014;24:3933–43.
-
- Zilberman M, Egozi D, Shemesh M. et al. Hybrid wound dressings with controlled release of antibiotics: structure-release profile effects and in vivo study in a guinea pig burn model. Acta Biomater. 2015;22:155–63. - PubMed
-
- Chester D, Brown AC. The role of biophysical properties of provisional matrix proteins in wound repair. Matrix Biol. 2017;60-61:124–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

