Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization
- PMID: 30429892
- PMCID: PMC6217052
- DOI: 10.7150/thno.27760
Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization
Abstract
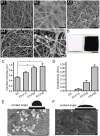
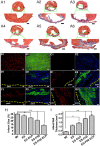
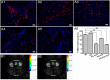
The controversy between polypyrrole's (Ppy) biocompatibility and its aggregation on nanofibers impedes application of conductive Ppy-incorporated nanofibers to create engineered cardiac microenvironments. The purpose of this study was to fabricate a functional scaffold for engineering cardiac patches (ECP) using a high concentration of methyl acrylic anhydride-gelatin (GelMA)-Ppy nanoparticles, mussel-inspired crosslinker, and electrospun (ES)-GelMA/polycaprolactone (PCL) nanofibrous membrane. Methods: First, spherical GelMA-Ppy nanoparticles were obtained when the methacrylate groups of GelMA formed a self-crosslinked network through oxidative polymerization of Ppy. Second, GelMA-Ppy nanoparticles were uniformly crosslinked on the ES-GelMA/PCL membrane through mussel-inspired dopamine-N'N'-methylene-bis-acrylamide (dopamine-MBA) crosslinker. Finally, the feasibility of the dopa-based conductive functional ECP scaffold was investigated in vitro and in vivo. Results: The GelMA-Ppy nanoparticles displayed excellent biocompatibility at a high concentration of 50 mg/mL. The massive GelMA-Ppy nanoparticles could be uniformly distributed on the ES nanofibers through dopamine-MBA crosslinker without obvious aggregation. The high concentration of GelMA-Ppy nanoparticles produced high conductivity of the dopamine-based (dopa-based) conductive membrane, which enhanced the function of cardiomyocytes (CMs) and yielded their synchronous contraction. GelMA-Ppy nanoparticles could also modify the topography of the pristine ES-GelMA/PCL membrane to promote vascularization in vitro. Following transplantation of the conductive membrane-derived ECP on the infarcted heart for 4 weeks, the infarct area was decreased by about 50%, the left ventricular shortening fraction percent (LVFS%) was increased by about 20%, and the neovascular density in the infarct area was significantly increased by about 9 times compared with that in the MI group. Conclusion: Our study reported a facile and effective approach to developing a functional ECP that was based on a mussel-inspired conductive nanofibrous membrane. This functional ECP could repair infarct myocardium through enhancing cardiac function and revascularization.
Keywords: dopamine; electrospun membrane; myocardial infarction; polypyrrole nanoparticles; revascularization.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







References
-
- Navaei A, Saini H, Christenson W, Sullivan RT, Ros R, Nikkhah M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016;41:133–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

