Porphyrins as ligands for 64copper: background and trends
- PMID: 30429966
- PMCID: PMC6194497
- DOI: 10.1039/c8md00263k
Porphyrins as ligands for 64copper: background and trends
Abstract
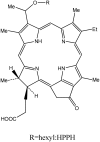
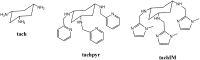
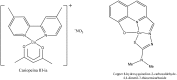
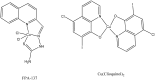
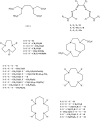
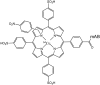
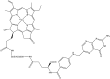
Porphyrins and 64Cu have emerged as a novel synergic option for applications in PET molecular imaging. Both the characteristics and photophysical properties of macrocyclic porphyrins and the relatively long half-life of the copper isotope, in addition to the increased tumor-specific uptake of porphyrins compared to normal cells, make this complex an attractive option not only for diagnosis but also for therapeutic applications. Herein, we present an overview of the latest results on the development of PET agents based on porphyrins and 64Cu, including methods used to improve the selectivity of these macrocycles when conjugated with biological units such as monoclonal antibodies, peptides or proteins.
Figures






Similar articles
-
Development of Novel PSMA Ligands for Imaging and Therapy with Copper Isotopes.J Nucl Med. 2020 Jan;61(1):70-79. doi: 10.2967/jnumed.119.229054. Epub 2019 Sep 20. J Nucl Med. 2020. PMID: 31541034
-
Increasing Reaction Rates of Water-Soluble Porphyrins for 64Cu Radiopharmaceutical Labeling.Molecules. 2023 Mar 3;28(5):2350. doi: 10.3390/molecules28052350. Molecules. 2023. PMID: 36903596 Free PMC article.
-
In vivo behavior of copper-64-labeled methanephosphonate tetraaza macrocyclic ligands.J Biol Inorg Chem. 2003 Jan;8(1-2):217-25. doi: 10.1007/s00775-002-0408-5. Epub 2002 Oct 11. J Biol Inorg Chem. 2003. PMID: 12459917
-
Porphyrins and related macrocycles: Combining photosensitization with radio- or optical-imaging for next generation theranostic agents.Photodiagnosis Photodyn Ther. 2018 Sep;23:281-294. doi: 10.1016/j.pdpdt.2018.06.023. Epub 2018 Jul 29. Photodiagnosis Photodyn Ther. 2018. PMID: 30009949 Review.
-
Synthetic approaches for the conjugation of porphyrins and related macrocycles to peptides and proteins.Photochem Photobiol Sci. 2011 May;10(5):759-91. doi: 10.1039/c0pp00366b. Epub 2011 Apr 1. Photochem Photobiol Sci. 2011. PMID: 21455536 Review.
Cited by
-
Porphyrins as Chelating Agents for Molecular Imaging in Nuclear Medicine.Molecules. 2022 May 21;27(10):3311. doi: 10.3390/molecules27103311. Molecules. 2022. PMID: 35630788 Free PMC article. Review.
-
Quantitative Pharmacokinetics Reveal Impact of Lipid Composition on Microbubble and Nanoprogeny Shell Fate.Adv Sci (Weinh). 2024 Jan;11(4):e2304453. doi: 10.1002/advs.202304453. Epub 2023 Nov 30. Adv Sci (Weinh). 2024. PMID: 38032129 Free PMC article.
-
Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer.Theranostics. 2021 Aug 26;11(18):9054-9088. doi: 10.7150/thno.62479. eCollection 2021. Theranostics. 2021. PMID: 34522227 Free PMC article. Review.
-
The Antimicrobial Efficacy of Copper Complexes: A Review.Antibiotics (Basel). 2025 May 16;14(5):516. doi: 10.3390/antibiotics14050516. Antibiotics (Basel). 2025. PMID: 40426582 Free PMC article. Review.
-
Radiocopper in Radiopharmacy and Medical Use: Current Status and Perspective.J Med Chem. 2025 Feb 13;68(3):2356-2376. doi: 10.1021/acs.jmedchem.4c02885. Epub 2025 Feb 2. J Med Chem. 2025. PMID: 39895089 Free PMC article. Review.
References
-
- Vahidfar N., Jalilian A. R. Recent Patents and Topics on Imaging. 2005;5:3–12.
-
- Hambright P., Fawwaz R., Valk P., McRae J., Bearden A. J. Bioinorg. Chem. 1975;5:87–92. - PubMed
-
- Lakouas K. D., Huglo D., Mordon S., Vermandel M. Photodiagn. Photodyn. Ther. 2017;18:236–243. - PubMed
-
- Mody D. T. J. Porphyrins Phthalocyanines. 2000;4:362–367.
-
- Nowakowska M., KKȩpczyńskipczyński M. J. Photochem. Photobiol., A. 1998;116:251–256.
Publication types
LinkOut - more resources
Full Text Sources

