Extended duration vancomycin in recurrent Clostridium difficile infection: a systematic review
- PMID: 30430009
- PMCID: PMC6210635
- DOI: 10.1177/2049936118798276
Extended duration vancomycin in recurrent Clostridium difficile infection: a systematic review
Abstract
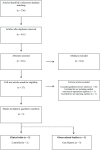
Clostridium difficile infections have a high recurrence rate following acute treatment. Extended duration vancomycin (EDV) is a mainstay for the treatment of recurrent Clostridium difficile infections (rCDI). Clinical disease guidelines recommend a variety of different vancomycin treatment regimens though based on weak, low-quality evidence. Patients typically receive an initial vancomycin treatment course of 7-14 days for the acute infection, followed by an extended duration vancomycin course. Multiple publications on the utility of EDV regimens have been published but few include reported effectiveness outcomes associated with a prescribed treatment regimen. The purpose of this review is to evaluate the safety and efficacy data on extended duration vancomycin regimens used in recurrent clostridium treatment. Five articles, three case series and two randomized open-label clinical trials, were identified which included both elements. Outcomes were evaluable in 174 patients, 31 from randomized trials, with prior average recurrent episodes ranging from 3 to 4. Vancomycin dose ranged from 3500 to >6800 mg with therapy durations extending from 21 days to over 60 days. Follow-up duration ranged from 10 weeks to 12 months. Case series reported success rates for EDV in rCDI from 61% to 100%, while randomized trials found lower success rates from 26% to 58%. Taper and pulse regimens reported superior outcomes compared to pulse-only regimens, 58-100% versus 26-81%, respectively. Comparative EDV data is limited. Current available data supports an EDV regimen which includes both a daily dosing taper followed by an every 48 or 72 h pulse.
Keywords: Clostridium difficile; pulse; recurrent; taper; vancomycin.
Conflict of interest statement
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Similar articles
-
Vancomycin Taper and Pulse Regimen With Careful Follow-up for Patients With Recurrent Clostridium difficile Infection.Clin Infect Dis. 2017 Oct 15;65(8):1396-1399. doi: 10.1093/cid/cix529. Clin Infect Dis. 2017. PMID: 28591789
-
The Clinical Efficacy, Safety, and Tolerability of Vancomycin for the Treatment of Recurrent Clostridioides difficile Infection - A Systematic Review.Drug Healthc Patient Saf. 2023 Mar 21;15:63-71. doi: 10.2147/DHPS.S348501. eCollection 2023. Drug Healthc Patient Saf. 2023. PMID: 36974197 Free PMC article. Review.
-
Pulsed dosing and extended daily dosing of oral vancomycin do not facilitate clearance of Clostridioides difficile colonization in mice.Antimicrob Agents Chemother. 2024 Jan 10;68(1):e0090323. doi: 10.1128/aac.00903-23. Epub 2023 Dec 14. Antimicrob Agents Chemother. 2024. PMID: 38095427 Free PMC article.
-
Oral Vancomycin Followed by Fecal Transplantation Versus Tapering Oral Vancomycin Treatment for Recurrent Clostridium difficile Infection: An Open-Label, Randomized Controlled Trial.Clin Infect Dis. 2017 Feb 1;64(3):265-271. doi: 10.1093/cid/ciw731. Epub 2016 Nov 9. Clin Infect Dis. 2017. PMID: 28011612 Clinical Trial.
-
Effective Dosage of Oral Vancomycin in Treatment for Initial Episode of Clostridioides difficile Infection: A Systematic Review and Meta-Analysis.Antibiotics (Basel). 2019 Oct 1;8(4):173. doi: 10.3390/antibiotics8040173. Antibiotics (Basel). 2019. PMID: 31581576 Free PMC article. Review.
Cited by
-
Economic evaluation of Faecal microbiota transplantation compared to antibiotics for the treatment of recurrent Clostridioides difficile infection.EClinicalMedicine. 2020 Jun 27;24:100420. doi: 10.1016/j.eclinm.2020.100420. eCollection 2020 Jul. EClinicalMedicine. 2020. PMID: 32637898 Free PMC article.
-
Updates in Treatment of Recurrent Clostridium difficile Infection.J Clin Med Res. 2019 Jul;11(7):465-471. doi: 10.14740/jocmr3854. Epub 2019 Jun 11. J Clin Med Res. 2019. PMID: 31236163 Free PMC article. Review.
References
-
- Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect 2009; 58: 403–410. - PubMed
-
- McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Disease Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66: e1–e48. DOI: 10.1093/cid/cix1085. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases