Rare Earth Doped Ceria: The Complex Connection Between Structure and Properties
- PMID: 30430105
- PMCID: PMC6220118
- DOI: 10.3389/fchem.2018.00526
Rare Earth Doped Ceria: The Complex Connection Between Structure and Properties
Abstract
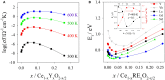
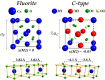
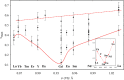
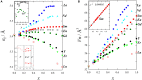
The need for high efficiency energy production, conversion, storage and transport is serving as a robust guide for the development of new materials. Materials with physical-chemical properties matching specific functions in devices are produced by suitably tuning the crystallographic- defect- and micro-structure of the involved phases. In this review, we discuss the case of Rare Earth doped Ceria. Due to their high oxygen diffusion coefficient at temperatures higher than ~500°C, they are very promising materials for several applications such as electrolytes for Solid Oxide Fuel and Electrolytic Cells (SOFC and SOEC, respectively). Defects are integral part of the conduction process, hence of the final application. As the fluorite structure of ceria is capable of accommodating a high concentration of lattice defects, the characterization and comprehension of such complex and highly defective materials involve expertise spanning from computational chemistry, physical chemistry, catalysis, electrochemistry, microscopy, spectroscopy, and crystallography. Results coming from different experimental and computational techniques will be reviewed, showing that structure determination (at different scale length) plays a pivotal role bridging theoretical calculation and physical properties of these complex materials.
Keywords: defects chemistry; diffraction; energy; microscopy; rare earths doped ceria; spectroscopy; structure; theoretical calculations.
Figures


References
-
- Acharya S. A., Gaikwad V. M., D'Souza S. W., Barman S. R. (2014). Gd/Sm dopant-modified oxidation state and defect generation in nano-ceria. Solid State Ionics 260, 21–29. 10.1016/j.ssi.2014.03.008 - DOI
-
- Adijanto L., Sampath A., Yu A. S., Cargnello M., Fornasiero P., Gorte R. J., et al. (2013). Synthesis and stability of Pd@CeO2 core–shell catalyst films in solid oxide fuel cell anodes. ACS Catal. 3, 1801–1809. 10.1021/cs4004112 - DOI
-
- Ahn K., Yoo D. S., Prasad D. H., Lee H. W., Chung Y. C., Lee J. H. (2012). Role of multivalent Pr in the formation and migration of oxygen vacancy in Pr-doped ceria: experimental and first-principles investigations. Chem. Mater. 24, 4261–4267. 10.1021/cm3022424 - DOI
-
- Ainscough J. B., Moore D. A., Osborn S. C. (1975). The kinetics of the C + B transformation in europium sesquioxide. J. Nucl. Metar. 55, 229–232. 10.1016/0022-3115(75)90156-7 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

