Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models
- PMID: 30430109
- PMCID: PMC6220074
- DOI: 10.3389/fbioe.2018.00154
Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models
Abstract
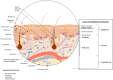
The relevance for in vitro three-dimensional (3D) tissue culture of skin has been present for almost a century. From using skin biopsies in organ culture, to vascularized organotypic full-thickness reconstructed human skin equivalents, in vitro tissue regeneration of 3D skin has reached a golden era. However, the reconstruction of 3D skin still has room to grow and develop. The need for reproducible methodology, physiological structures and tissue architecture, and perfusable vasculature are only recently becoming a reality, though the addition of more complex structures such as glands and tactile corpuscles require advanced technologies. In this review, we will discuss the current methodology for biofabrication of 3D skin models and highlight the advantages and disadvantages of the existing systems as well as emphasize how new techniques can aid in the production of a truly physiologically relevant skin construct for preclinical innovation.
Keywords: 3D tissue model; biofabrication; bioprinting; electrospinning; in vitro; preclinical testing; skin; skin disease.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

