Interaction between opposing modes of phospho-regulation of the proneural proteins Ascl1 and Ngn2
- PMID: 30430141
- PMCID: PMC6206610
- DOI: 10.12688/wellcomeopenres.14848.1
Interaction between opposing modes of phospho-regulation of the proneural proteins Ascl1 and Ngn2
Abstract
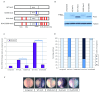
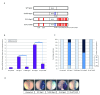
From the relatively simple nervous system of Drosophila to the elaborate mammalian cortex, neurogenesis requires exceptional spatial and temporal precision to co-ordinate progenitor cell proliferation and subsequent differentiation to a diverse range of neurons and glia. A limited number of transiently expressed proneural basic-helix-loop-helix (bHLH) transcription factors, for example achaete-scute-complex (as-c) and atonal (ato) in Drosophila and the vertebrate homologues Ascl1 and Neurogenin2 (Ngn2), are able to orchestrate the onset of neuronal determination, context-dependent subtype selection and even influence later aspects of neuronal migration and maturation. Within the last decade, two models have emerged to explain how the temporal activity of proneural determination factors is regulated by phosphorylation at distinct sites. One model describes how cell-cycle associated phosphorylation on multiple sites in the N and C termini of vertebrate proneural proteins limits neuronal differentiation in cycling progenitor cells. A second model describes phosphorylation on a single site in the bHLH domain of Drosophila atonal that acts as a binary switch, where phosphorylation terminates proneural activity. Here we combine activating mutations of phosphorylation sites in the N- and C- termini with an inhibitory phospho-mimetic mutation in the bHLH domain of Ascl1 and Ngn2 proteins, and test their functions in vivo using Xenopus embryos to determine which mode of phospho-regulation dominates. Enhancing activity by preventing N- and C terminal phosphorylation cannot overcome the inhibitory effect of mimicking phosphorylation of the bHLH domain. Thus we have established a hierarchy between these two modes of proneural protein control and suggest a model of temporal regulation for proneural protein activity.
Keywords: Ascl1; Neurogenin2; Proneural; Xenopus.; neurogenesis; phospho-regulation.
Conflict of interest statement
No competing interests were disclosed.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

