Lymph nodes as barriers to T-cell rejuvenation in aging mice and nonhuman primates
- PMID: 30430748
- PMCID: PMC6351843
- DOI: 10.1111/acel.12865
Lymph nodes as barriers to T-cell rejuvenation in aging mice and nonhuman primates
Abstract
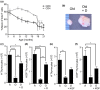
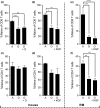
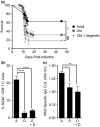
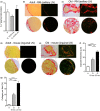
In youth, thymic involution curtails production of new naïve T cells, placing the onus of T-cell maintenance upon secondary lymphoid organs (SLO). This peripheral maintenance preserves the size of the T-cell pool for much of the lifespan, but wanes in the last third of life, leading to a dearth of naïve T cells in blood and SLO, and contributing to suboptimal immune defense. Both keratinocyte growth factor (KGF) and sex steroid ablation (SSA) have been shown to transiently increase the size and cellularity of the old thymus. It is less clear whether this increase can improve protection of old animals from infectious challenge. Here, we directly measured the extent to which thymic rejuvenation benefits the peripheral T-cell compartment of old mice and nonhuman primates. Following treatment of old animals with either KGF or SSA, we observed robust rejuvenation of thymic size and cellularity, and, in a reporter mouse model, an increase in recent thymic emigrants (RTE) in the blood. However, few RTE were found in the spleen and even fewer in the lymph nodes, and SSA-treated mice showed no improvement in immune defense against West Nile virus. In parallel, we found increased disorganization and fibrosis in old LN of both mice and nonhuman primates. These results suggest that SLO defects with aging can negate the effects of successful thymic rejuvenation in immune defense.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

